Search Results
Showing results for: additive manufacturing
Showing 50 Of 63 records
-
Global quality
At Stryker, quality is first in everything we do. Read our Quality Policy and learn more about how we build quality into our culture, our processes and our products.
-
Tritanium C
Anterior Cervical Cage
A hollow implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing
-
Tritanium TL
Curved Posterior Lumbar Cage
A hollow implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing
-
Tritanium PL
Posterior Lumbar Cage
A hollow, rectangular implant that consists of a unique configuration of both solid and porous structures built using AMagine Technology, our proprietary approach to implant creation using additive manufacturing
-
Acetabular System
Acetabular System
Have confidence in a powerful combination–a system that builds on the legacy that has defined our Trident brand for more than two decades, paired with the latest additive manufactured Tritanium In-Growth Technology or PureFix HA.
Trident II is created for case-to-case confidence that comes from building on a system backed by years of successful clinical data1-5 and reliable features such as the notable Trident Innerchange Locking Mechanism, Modular Dual Mobility (MDM), and our X3 precisely engineered polyethylene. -
Unique Device Identification (UDI)
The U.S. Food and Drug Administration (FDA) created unique device identification, often abbreviated UDI, a rule that requires medical device manufacturers to update their products with a unique device identifier that includes both device and production identifiers (such as expiration date and lot or serial number).
-
X3
Precisely engineered polyethylene
Our X3 polyethylene supports our total hip, knee and shoulder arthroplasty solutions. X3’s patented manufacturing process of sequential crosslinking uses three separate gamma irradiation doses with an annealing step after each.
-
Triathlon® Cementless – Fixation you can trust.
Fixation you can trust.
Triathlon Cementless combines the kinematics of Triathlon with the latest highly porous biologic fixation technology. With over one million cementless knees implanted to date1 and impressive survivorship data2-4 since it was introduced in 2013, Triathlon Cementless has become a trusted solution for surgeons across the globe.
-
Spine
We deliver the total spine experience, empowering our customers to keep the world moving. Together, we do more to challenge the standard, respond to clinical needs and pioneer new possibilities.
Our core technologies include biologic solutions, spinal implants, and surgical instruments for use in spine procedures. Our enabling technologies include digital health, guidance, and imaging for both cranial and spine procedures.
-
Knee
Implants
We offer knee replacement implants for partial and total knee arthroplasty for primary and revision procedures. Our implants feature our flagship cemented and cementless TKA solution, the Triathlon Knee System.
-
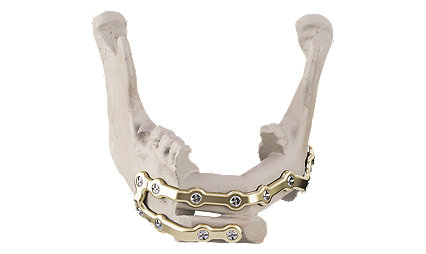
Facial iD - Mandible Reconstruction
A patient specific solution
Rigid internal fixation for primary and secondary mandible reconstruction.
-
CR Reports and Resources
ESG Resource hub
Access Stryker’s environmental, social and governance reports, data and resources
-
Environmental programs
Climate and the environment
Environmental programs, processes and initiatives to drive sustainability
-
ProCare Services | Communications Business
You focus on your patients and staff; we have your equipment covered. ProCare is your trusted partner, offering flexible service solutions to maximize uptime without compromising safety. As the original equipment manufacturer, we provide unmatched service with original Stryker parts, proprietary product knowledge, and expert service professionals—setting the gold standard in equipment care.
-
ProCare Services | Instruments
You focus on your patients and staff; we have your equipment covered. ProCare is your trusted partner, offering flexible service solutions to maximize uptime without compromising safety. As the original equipment manufacturer, we provide unmatched service with original Stryker parts, proprietary product knowledge, and expert service professionals—setting the gold standard in equipment care.
-
Monterey AL Interbody System
Fixated on science
With a design to enhance the ability to create indirect decompression, a Tritanium structure to facilitate bone in-growth [1], and an array of footprints, heights and lordotic options — our Monterey AL Interbody System aims to deliver on all fronts. Plus, our instrumentation is designed to support your surgical style whether it’s freehand, partially guided or fully guided. Here’s a look at the careful crafting we do to help meet your surgical needs and personal preferences.
-
Direct Superior Approach
Superior soft tissue preservation
Direct Superior approach is an iliotibial band and muscle-sparing surgical approach to the hip that offers the potential benefits to the patient of faster recovery, less pain and greater satisfaction.
-
Chesapeake Cervical-Ti
Stabilization System
The Chesapeake Cervical-Ti Stabilization System is a multi-screw construct designed to provide stability to the anterior column while eliminating the need for supplemental fixation in the cervical spine. [1] Featuring SMARTLock Technology, the system exemplifies advances in new and innovative instrumentation.
-
Stryker boosts innovation: R&D expands with medical device testing facility
Stryker's Global Technology Centre (SGTC) has recently expanded to accommodate rigorous life cycle testing of medical devices, adding highly skilled engineers and microbiologists to conduct expanded testing.
-
Chesapeake Anterior-Lumbar
Stabilization System
The Chesapeake Anterior-Lumbar Stabilization System is a multi-screw construct that is designed to provide stability to the anterior column, while reducing the need for supplemental fixation. [1] This system offers a comprehensive range of sizes carefully designed to assist with the restoration of sagittal balance and disc height. Featuring tifix Locking Technology, Chesapeake allows for appropriate implant placement required to achieve fusion.
-
Universal Orthognathic
1.7/2.0/2.3 mm rigid internal fixation system
Comprehensive fixation for orthognathic procedures.
-
Powered Instruments and Caregiver Safety
Perioperative Nurses | Sterile Processing Personnel | 3 contact hours
-
News archive
2013
-
Ultrasonic HARMONIC scalpel used for harmonic surgery
Reprocessed Ultrasonic Vessel Sealer/Divider
Reprocessed HARMONIC Scalpel solutions by Stryker for secure vessel sealing in general, pediatric, gynecologic, urologic, and thoracic surgery.
-
Operon D860 Surgical Tables
Surgical Table
The Operon D860 surgical table is designed to deliver on the increasing demand for flexibility, while providing for an optimal range of applications with its improved mobility and modular design.
-
VSP® Reconstruction
A digital planning solution for maxillofacial surgical procedures.


















?$preset_426_254$)





?$preset_426_254$)





