Search Results
Showing results for: order
Showing 50 Of 130 records
-
Contact information
Contact Stryker Instruments Teams. Connect with Customer service for help with placing new orders, make changes to accounts, connect with sales reps. Connect with Repairs to Call in Depot Repairs on Saws Drills and Consoles, or get replacements on Helmets, Attachments, batteries, and other accessories. Connect with Tech Support to request Field Repairs and Dispatches, Technical support on all Stryker instruments products, and support documentation like Instructions for use, medical safety data sheets, and care instructions. See below for contact numbers and emails.
-
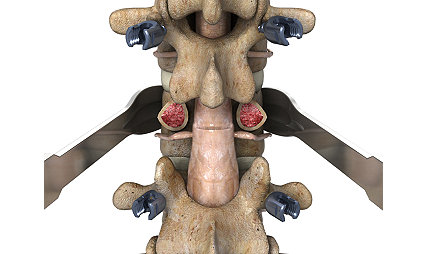
Hallux Interphalangeal Joint (IPJ) Arthrodesis
Arthrodesis of the great toe’s interphalangeal joint is a common procedure in order to treat a variety of conditions.
-
Hotel staff | handsfree device communication
The lightweight, hands-free Smartbadge brings together communication, work-order ticket management and a wearable panic button in one device. For staff who don’t need to manage work-order tickets, the wearable Minibadge allows the same ease of communication with a built-in panic button. Our devices can be worn discreetly underneath a coat or uniform and used with or without a wired or Bluetooth headset.
-
Spine Medical Education
We deliver world class education and training on the use of our products in accordance with their validated surgical technique. This is our enduring commitment to healthcare professionals and improving patient outcomes in order to make healthcare better.
-
Save Simply Sage Prevalon® MATS
New and reprocessed mobile air lateral transfer systems
We partner with Stryker Sage to offer a blended Prevalon MATS program offering a mixture of both new and reprocessed Prevalon MATS products at one low price.
- • One vendor to order from
- • One hundred percent fill rate
- • Optimized collections
- • Lower total facility cost
- • Divert unnecessary landfill waste
-
Save Simply Philips ECG
New and reprocessed 3 lead, 5 lead, and 12 lead sets, cables and adapters
We partner with Philips to offer a blended ECG program offering a mixture of both new and reprocessed ECG products at one low price through your Stryker Sustainability Solutions representative.
- • One vendor to order from
- • One part number for new and reprocessed
- • One hundred percent fill rate
- • Predictable savings
- • Divert unnecessary landfill waste
-
Biologics
Stryker’s advantage
Stryker is one of the world’s leading medical technology companies. In order to better partner with our customers, we made the decision to invest in the development of our biologics portfolio to bring a comprehensive solution to a variety of specialties, including orthopaedic, spine, sports medicine, and CMF.
Stryker continues to develop innovative technologies to enhance our spectrum of products, while collaborating with industry-leading tissue organizations to offer a robust portfolio of proprietary spinal grafts, viable bone matrices, demineralized bone matrix products, synthetic bone grafting substitutes, amniotic and dermal membranes, and nerve products.
Our focus on actively bringing new and compelling products to our portfolio is guided by our commitment to both surgeon and patient needs, as well as a quality-first foundation. Stryker works with various heath authorities and regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and organizations like the American Association of Tissue Banks (AATB) to ensure our products meet the appropriate standards. -
Unique Device Identification (UDI)
The U.S. Food and Drug Administration (FDA) created unique device identification, often abbreviated UDI, a rule that requires medical device manufacturers to update their products with a unique device identifier that includes both device and production identifiers (such as expiration date and lot or serial number).
-
The Sync Badge is here – hands-free, wearable hospital communication
Quick, reliable hands-free communication. Workflow efficiency. Dedicated panic button. All in one wearable device.
-
Room design
Reimagine your OR.
The Meta Quest 3, together with ByDesign AR, brings your OR design to life as you are able to walk around the room and visually validate the space using mixed reality.
-
Reprocessing and remanufacturing in healthcare
Reprocessing of single-use medical devices (SUDs) is the practice of inspecting, cleaning, function testing, sterilizing and packaging so that they can be clinically and safely used again. Reprocessing of SUDs is regulated by the FDA. We satisfy all FDA regulatory requirements to reprocess medical devices and ensure that our reprocessed devices are substantially equivalent to original equipment devices.
-
ProCare Services
Image Guided Therapies
-
Tensix
Demineralized Bone Graft
Tensix is a ready-to-use line of demineralized bone matrix (DBM) bone grafting putty available in a convenient pre-hydrated, ambient temperature stored package.
-
Shoulder iD™
Primary Reversed Glenoid
Personalized glenoid implant powered by Blueprint for your patient’s unique anatomy
-
IBD PEEKᶜ
Anterior Cervical Spacer System
A sterile packaged anterior cervical spacer that offers a chamfered leading edge, a large open graft window and a collet-style inserter
-
Osteotomy
Instrument Set
Comprehensive set of instruments for use in pedicle subtraction osteotomies and vertebral column resections
-
Tornier Humeral Nail
Humeral Fracture System
Intramedullary nail for treating various types of proximal and diaphyseal fractures of the humerus.
-
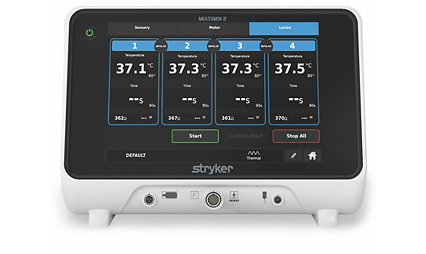
MultiGen 2 radiofrequency generator
Together, the MultiGen 2 RF generator and Venom cannula and electrode system delivers large, minimally invasive lesions in a short amount of time. Made for reliability, efficiency and ultimate control and customization, our Performance Platform delivers an advantage for you and your patients.
-
Masimo RD SET
Reprocessed SP02 sensors
For over a decade, Stryker’s Sustainability Solutions has remained the global leader in reprocessing and the third largest domestic manufacturer of single-use pulse oximeter sensors. Our reprocessed RD SET sensors are available in both woven and transpore styles for adult, pediatric and infant patients.
-
Powered Instruments and Caregiver Safety
Perioperative Nurses | Sterile Processing Personnel | 3 contact hours
-
AXS Catalyst 5
Distal Access Catheter
The CAT 5 Distal Access Catheter provides navigation and support for distal neurovascular access cases.
-
Nellcor™ OxiMax™ pulse oximeter sensors
Reprocessed SP02 sensors
The Nellcor OxiMax pulse oximeter sensors are noninvasive devices attached to a patient’s fingertip, toe or foot and emits light through the appendage to consistently monitor blood oxygen saturation. Stryker's Sustainability Solutions offers single use, reprocessed Nellcor OxiMax pulse oximeter devices in both transpore and woven styles for adult, pediatric, infant and neonatal patients.
-
Fuseforce
Fixation System
The Fuseforce Fixation System provides dynamically compressive nitinol staples packaged together with all corresponding single-use sterile surgical instruments. Nine unique sizes are available from 8 to 25mm. Wright Medical Group - Stryker
-
Reprocessed Masimo LNCS® Pulse Oximeter Sensors: sizes and designs
Reprocessed SP02 sensors
Masimo LNCS pulse oximeter sensors designed for short or long-term patient monitoring. Offers a variety of traditional cable lengths and connector positions.
-
Survivor: Mark Dundore
Mark, who has had atrial fibrillation (Afib) since 2009, began to feel unwell and had difficulty breathing after lunch. Once Mark became unresponsive, coworkers began chest compressions and used an AED they installed just three weeks prior. The presence of this device on-site helped saved Mark's life.