Medical device reprocessing adds value to hospitals by aligning with their internal goals. As the trusted supplier of choice for roughly 3,500 U.S. hospitals, including nearly all of U.S. News & World Report’s “Best Hospitals,” our approach is simple: quality first, savings always. In 2023, Stryker's Sustainability Solutions helped their customers divert more than 5 million pounds of waste from the landfill through our reprocessing programs, saving 3,250 customers approximately $238 million. In the last five years, we have helped nearly 50 Integrated Delivery Networks save over $1 million annually in supply chain costs.
Stryker’s Sustainability Solutions OnTrack program management provides custom solutions to optimize financial and environmental sustainability. Reprocessing success requires a focus on all the critical savings performance drivers: culture, contracting, clinical engagement, purchasing/logistics, staff awareness and education. Our OnTrack program management provides our customers with a true partnership to achieve those goals.
Watch the life of a reprocessed EP catheter
Featured products
Learn more about the single-use devices that SSS reprocesses.
MyoSure® Hysteroscopy Devices: Sustainable Design
The reprocessed Hologic MyoSure® hysteroscopic tissue...
More
Collections
Devices are collected at the hospital utilizing Stryker’s advanced collection system. Containers are placed in convenient usage areas and are regularly shipped by Stryker staff to our reprocessing facility.

Sorting
Once collection containers have arrived at our reprocessing facility, contents are sorted to isolate reprocessable devices from devices that cannot be reprocessed.

Reprocessing
Devices are cleaned and disassembled by our highly trained staff. Parts are individually inspected and, depending on the device, may be replaced. Some devices go through more elaborate reprocessing procedures.
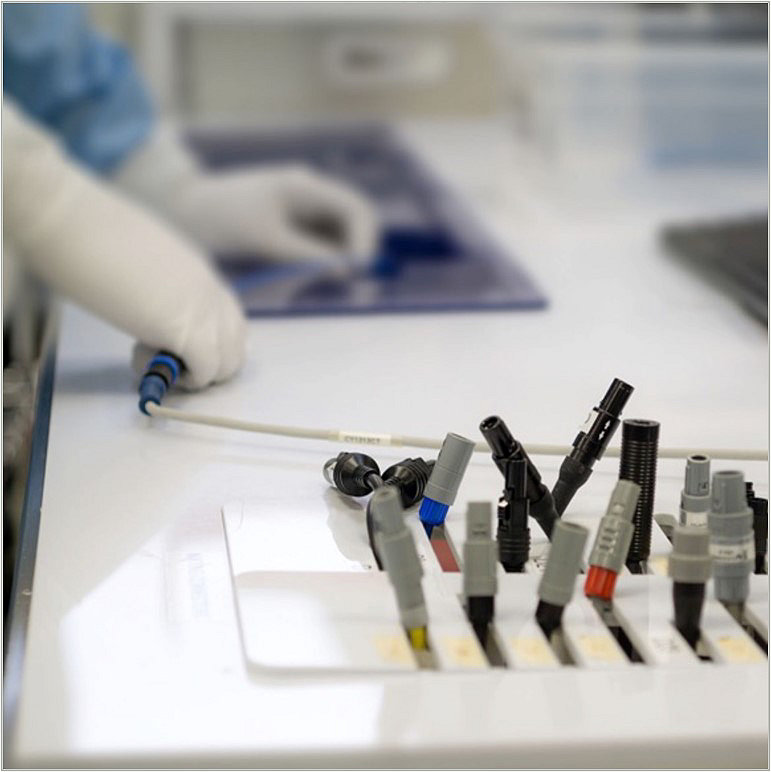
Inspection
Once rebuilt, devices are inspected and function tested based on validated processes. Most devices are then sterilized, repackaged, labeled and shipped back to the customer.

Purchasing
Hospitals can monitor how many devices we have collected and are available to buy-back through our repaid return order generation process. Based on this, they order what they need from reprocessed inventories, before they order devices from the original manufacturer – this decreases purchasing costs.

Receiving
Devices are shipped in original manufacturer case quantities to hospitals in clearly marked boxes and placed in the supply room next to original manufacturer devices. When pulling devices for cases, most of our customers pull reprocessed devices before selecting new ones so they can realize critical savings.
Connect with Stryker
Follow SSS on LinkedIn, Instagram and Twitter for the latest news and events.
CORPM-GSNPS-SYK-1910250


