2018 Highlights
and Benjamin Saving a life ... and a way of living.

Boris, who is 71, suddenly stopped talking during the drive. When Benjamin realized something was wrong with his grandfather, he grabbed the steering wheel to avoid crashing and guided the car to the side of the highway to call 911.
“I started to scream and scream, and he didn’t respond at all,” Benjamin recalls. “I was scared, so I called my mom and she told me to call 911. I pressed all the buttons, and I’m not sure what stopped the car.”
“I lost everything. I couldn’t hear anything, and I could only see from the left side,” says Boris.
An ambulance arrived to take him to UMass Memorial Medical Center in Worcester, MA, where he was seen by Dr. Ajit Puri, an experienced neurointervention specialist who uses minimally invasive techniques to treat stroke, brain aneurysms and carotid stenosis.
“Boris had a massive stroke where the main artery of the brain, called the middle cerebral artery, was blocked by a big clot,” says Dr. Puri. “We call that a large vessel occlusion. He could not move the entire right side of his body. He could not speak at all, and he was having a severe gaze preference,” which indicates eye movement limitation.
Boris’s best chance for recovery with a large vessel occlusion was to have the clot removed using a stent retriever, a minimally invasive procedure that is now the standard of care for large vessel blockages. Dr. Puri guided Stryker’s Trevo Retriever from Boris’s femoral artery in his leg up to the brain, where the retriever ensnared the clot. Dr. Puri was then able to remove the retriever with the clot back through a catheter.
With Dr. Puri’s extensive experience using the stent retriever, the procedure, from the time the catheter was inserted, only took 21 minutes to restore blood flow to the brain. As a result, Boris was soon able to move the right side of his body and his speech is now back to normal.
“I feel lucky, because if it weren’t for Ben, if I was alone in the car—I’m pretty sure I was going 65 miles an hour—I would crash somewhere,” says Boris.
Every second counts
Stroke is the fifth leading cause of death in the U.S. “Getting emergency help as soon as you recognize the signs of stroke for you or a loved one is critical,” says Dr. Puri. In fact, patients can lose nearly two million brain neurons every minute until the clot is removed.
Boris was able to avoid long-term brain damage, thanks to Benjamin’s actions and the quick response at the hospital, including the use of advanced treatments like the Trevo Retriever.
Until recently, the use of a stent retriever to remove a clot was limited to the first six hours after an ischemic stroke, which meant patients seeking help outside of this time window faced severe disability and even death. In early 2018 Stryker’s Trevo Retriever became the first and only thrombectomy device to receive FDA clearance to significantly reduce disability in patients up to 24 hours from symptom onset. To learn more about the impact of these changes visit Stryker.com/Trevo24.
Dr. Puri saved my way of living."

Boris Hutorin and his grandson, Benjamin

Dr. Ajit Puri, Neurointervention specialist, and Chris Lear, Stryker Territory Manager, Neurovascular
Trevo Retriever: around the globe
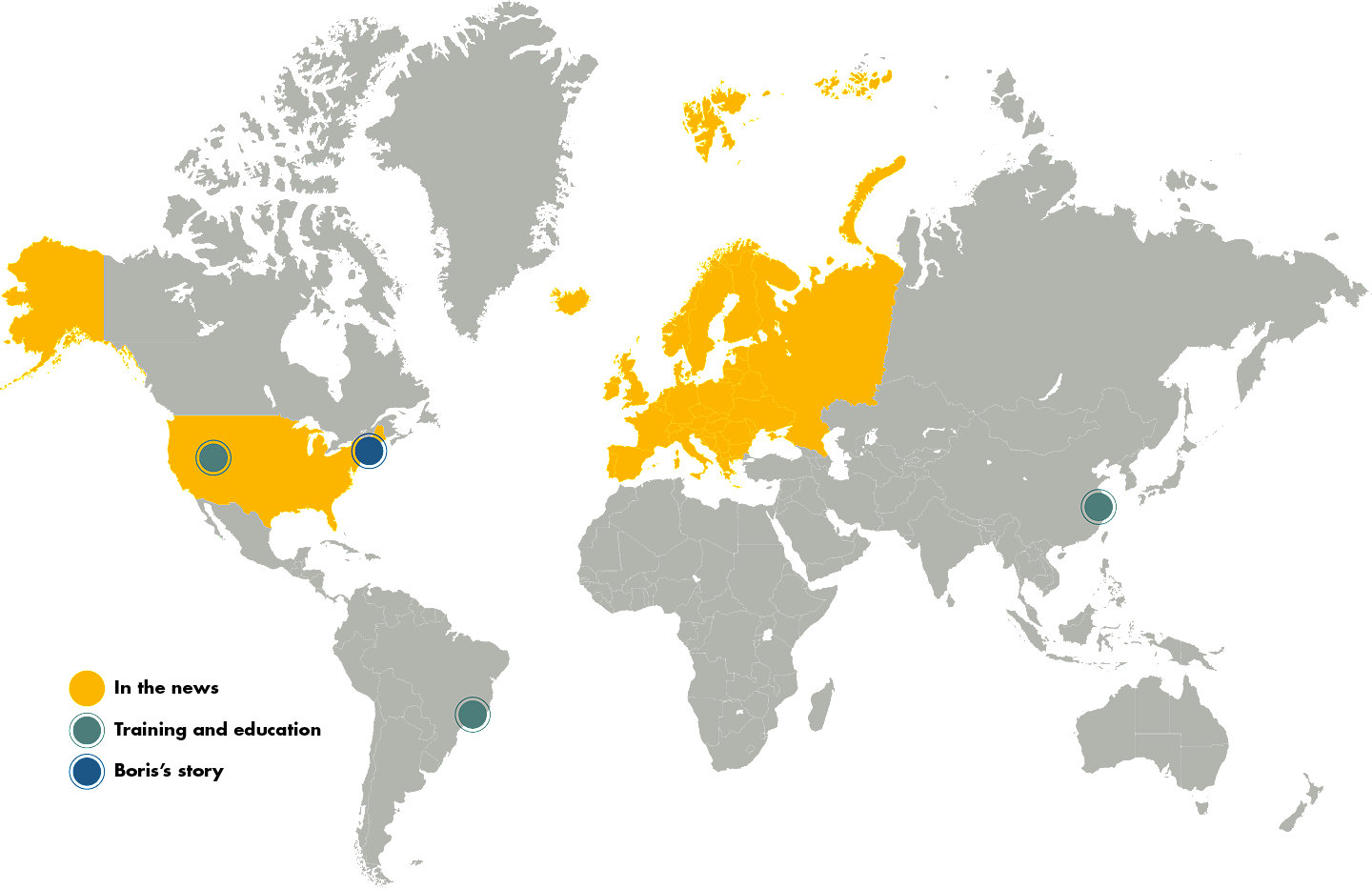
From left to right
Salt Lake City Learning Center and Trevo manufacturing
Utah
Trevo Retriever receives expanded indication for stroke treatment up to 24 hours in U.S.
February 2018
Boris survives his ischemic stroke thanks to grandson Benjamin and Dr. Ajit Puri
Massachusetts
Ischemic stroke training workshop
Brazil
Trevo Retriever receives expanded indication for stroke treatment up to 24 hours in Europe
May 2018
Suzhou Training Center
China
Mergers and acquisitions have long been a priority for our capital allocation and an accelerator of long-term performance. Our ability not only to acquire the right businesses but also to integrate them quickly to drive growth is a strength.
In 2018, we completed a series of acquisitions that will strengthen our core business and add complementary products where we already have a market presence.

Hygia Health Services
With a focus on reprocessing patient-care single-use devices like ECG leads and infusor bags, Hygia will broaden the reprocessing capabilities of our Sustainability Solutions business. A premier provider of sustainability solutions in the global healthcare market, Hygia has helped several thousand single-use device reprocessing customers collectively save millions of dollars in supply costs and divert millions of pounds of waste from landfills.

VEXIM
We acquired this French medical device company that specializes in the minimally invasive treatment of vertebral compression fractures. VEXIM’s flagship product is the SpineJack system, a mechanical expandable vertebral compression fracture implant for fracture reduction and stabilization. VEXIM’s portfolio is highly complementary to the Interventional Spine (IVS) business of our Instruments division.
Entellus
This high-growth global medical technology company focuses on minimally invasive devices that enable physicians to conveniently and comfortably perform a broad range of Ear, Nose and Throat (ENT) procedures, including the treatment of nasal airway obstructions. As a leader in the ENT segment, Entellus brings a comprehensive portfolio of products that deliver superior patient and physician experiences.
HyperBranch
Supporting our growth strategy within our Neurotechnology business, HyperBranch develops and markets products for dural repair. The addition of its Adherus AutoSpray dural sealant to our cranial closure portfolio strengthens our position of excellence in the dural repair space.

K2M
K2M is a global leader of complex spine and minimally invasive products designed to achieve three-dimensional Total Body Balance. Spine surgeons use these technologies and techniques to treat some of the most complicated spinal pathologies. K2M’s innovative product portfolio and robust pipeline strengthen our leadership in the spine and neurotechnology markets, while expanding our global footprint.

Invuity
The leader in advanced photonics and single-use, lighted instruments delivers enhanced visualization for a wide variety of clinical applications, serving a number of segments, including orthopaedic and spine surgery, general surgery and women’s health procedures. These innovative products, which are market leaders in illumination to help make surgery more precise, efficient and safe, are highly complementary to our Instruments business.
Mako Technology has evolved during its more than 13-year history. The year 2018 marked our five-year acquisition anniversary of MAKO Surgical Corp., and through the end of 2018 more than 200,000 Mako procedures have been performed in 26 countries across the globe.1
Mako Robotic-Arm Assisted Surgery enables surgeons to have a more predictable surgical experience through three unique steps: CT based enhanced planning, dynamic joint balancing and haptic guidance.
In partial knee and total hip applications, Mako has been shown to facilitate more accurate positioning to plan2,3 and has demonstrated enhanced patient-reported outcomes, like less pain than those undergoing manual surgery during the 90-day post-operative period for Mako Partial Knee, and significantly higher modified Harris Hip scores (92.1+/-10.5 vs. 86.1+/-16.2, p=0.002) for Mako Total Hip compared to manual surgery.4,5
Mako Total Knee was evaluated in two distinct, prospective, consecutive series, single-surgeon studies comparing patients undergoing conventional jig-based total-knee replacement versus Mako Total Knee surgery (40 patients6 and 30 patients7 in each cohort).

1. Stryker’s sales/case data.
2. Bell SW; Anthony I; Jones B; MacLean A; Rowe P; Blyth M. Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty: data from a prospective, randomized controlled study. J Bone and Joint Surg. 2016;98: 627–35.
3. Elson L, Dounchis J, Illgen R, Marchand R, et al. Precision of acetabular cup placement in robotic integrated total hip arthroplasty. Hip Int 2015; 25(6): 531–536.
4. Blyth MJ, Anthony I, Rowe P, Banger MS, MacLean A, Jones B. Robotic-arm assisted versus conventional unicompartmental knee arthroplasty: Exploratory secondary analysis of a randomized controlled trial. Bone and Joint Research. 2017 Nov 16 (11): 631.
5. Bukowski BR, Chughtai M, Anderson P, et al. Improved functional outcomes with robotic compared with manual total hip arthroplasty. Surg Technol Int. 2016 Oct.
6. Haddad FS, et al. Robotic-arm assisted total knee arthroplasty is associated with improved early functional recovery and reduced time to hospital discharge compared with conventional jig-based total knee arthroplasty. The Bone & Joint Journal, July 2018.
7. Haddad FS, et al. Iatrogenic Bone and Soft Tissue Trauma in Robotic-Arm Assisted Total Knee Arthroplasty Compared With Conventional Jig-Based Total Knee Arthroplasty: A Prospective Cohort Study and Validation of a New Classification System. J Arthroplasty. 2018 Aug;33(8):2496–2501. Epub 2018 Mar 27.
Professor Fares S. Haddad is a consultant of Stryker. However, Dr. Haddad and the authors of publications #6 and #7 did not receive financial or in-kind compensation for the research or publications.

building knowledge and experience
With a strong commitment to education and training for healthcare providers—to make healthcare better for patients—we have recently opened three state-of-the-art education and training centers.

Amsterdam Skills Centre
The Amsterdam Skills Centre (ASC), which opened its doors in February 2019, offers medical specialists and other healthcare professionals from all over the world an innovative learning environment. A collaboration of Amsterdam University’s Academic Medical Center and Stryker, which invested in the building and equipment, the ASC features 12 high-tech operating rooms, operating robots, virtual reality simulators and educational facilities. Amsterdam, The Netherlands
Asia Pacific Training Center
Our new Asia Pacific Training Center in Hong Kong will provide surgeons in the region with world-class training and simulated operations using the Mako Robotic-Arm Assisted system. The 6,000-square-foot space will also be a top venue for seminars, workshops and hands-on training in surgical education. Through the center, surgeons can strengthen their surgical competencies and share knowledge of the latest techniques in robotic surgery. More than 100 guests were on hand to celebrate the opening in October 2018. Hong Kong, China

Mako Education Center
The Mako Education Center is a premier training facility for surgeons, with a primary focus on Mako Robotic-Arm Assisted Surgery training and education. The 45,000-square-foot training center features a custom wet lab that accommodates 12 Mako stations, reflecting the importance of cutting-edge technology in medical and surgical applications. Following the ribbon-cutting, our Chairman and CEO, Kevin Lobo, hosted a global town hall for Stryker’s more than 200 team members in the area. Ft. Lauderdale, Florida, U.S.
That approach began with our founder, Dr. Homer Stryker, an orthopaedic surgeon in Kalamazoo, Michigan, who invented products in the local community hospital where he worked to better serve the needs of his patients.
This spirit of innovation continues to drive us today. We collaborate with hospitals and healthcare providers to make sure we understand their practice environment, treatment protocols and unmet needs.
Our deep understanding of our customers helps us design and manufacture products and services that make a difference for them.
We collaborate across the company and leverage our talented innovators to develop meaningful technology and solutions. And we recognize our best.
Our R&D Fellows Award recognizes significant accomplishments in research and development over time, reflecting the importance of innovation and collaboration to help solve customer problems and develop products and services that advance our mission.
Nominated by their R&D leader and division president, Fellows have developed breakthrough technology and market-leading products that have influenced the direction of our business.

John Scanlan
Senior Director, Business and Process Development, Advanced Operations Early in his career, John played a key role in developing and introducing many new knee, hip and bone cement products before leading teams in the development of our additive manufacturing technology. His work included technical and process design as well as the creation of a software and equipment supplier base for producing high-quality, innovative additive products. John’s leadership resulted in the launch of our first additive implant, the Triathlon Tritanium Tibial Baseplate, in June 2013, and many others that followed.

Robert Cohen
VP, R&D, Chief Technology Officer, Joint Replacement Robert’s many contributions to the advancement of hip and knee products over the years are evident in the 28 patents for which he is responsible, including the tibial component delta fit keel design, which is integral to tibial component stability and initial fixation. Robert was also instrumental in transitioning Mako robotics from being a niche player in partial knee replacement to establishing a broad-based arthroplasty platform, while guiding the development process and regulatory strategy for Mako Total Knee.
Inspiring women in STEM
We understand that the future of medical device innovation relies on exceptional talent in Science, Technology, Engineering and Math (STEM). That’s why our Stryker’s Women’s Network (SWN) partnered with I WISH (Inspiring Women in STEM), an initiative to inspire and motivate young female students to pursue careers in STEM. I WISH encourages female role models to share their experiences, from school subject choices and college and career choices to day-to-day work as engineers.
The statistics tell the story: 58 percent of students earning a degree in the United States are women, but only 39 percent of students earning STEM degrees are women.
I WISH hosted a conference and interactive exhibitions in Cork and Dublin, Ireland, where a Stryker team met with 6,000 women to show them how we are driven to make healthcare better.
Our team members also spoke to the teachers in attendance about our graduate programs and the opportunities that are available to students, including the Quality Graduate Rotational Program, the RISE Engineering Program, the Global Engineering Development Program and various internships.
We also had interactive, hands-on demonstrations of our products and technologies that improve and save lives, including defibrillators, endoscopes, cranial drills, biomaterials, stents, catheters, ultrasonics and robots, among others.
Employee resource groups (ERGs)
SWN enhances opportunities for members through networking, professional development, leadership messages, community outreach and educational projects that drive the attraction, development, engagement and retention of talented women. The network is open to all employees, both men and women, and includes thousands of members across the globe.
Other Stryker ERGs include Stryker’s Allies for Equality (SAFE), Women in Stryker’s Engineering (WISE), Stryker’s African American Network (SAAN), SOMOS (Hispanic/Latino), Stryker’s Emerging Professionals (SEP) and Stryker’s Veterans Association (SVA).
We understand that attracting and empowering a diverse team is critical for our business and we take steps to ensure that we have a great workplace for everyone.
"Gender balance increases our ability to attract the best talent and make healthcare better for customers and patients."
— Katherine Owen, Vice President,
Strategy and Investor Relations
FORTUNE World’s Most Admired Companies
#3 in 2019
Medical Products & Equipment
recipient for the 17th consecutive year
FORTUNE Change the World
#25 in 2018
Out of 57 companies — recipient for the first time
LinkedIn Top Companies
Where the World Wants to Work Now
#30 in 2018
recipient for the second time
Women’s Choice Awards
2018 awards
named to three lists including 100 Best Workplaces for Women, 100 Best Companies for Millennial Women and 50 Best Companies for Multicultural Women
FORTUNE World’s Best Workplaces
#12 in 2018
out of 25 companies — recipient for the second time
FORTUNE Best Workplaces (U.S.)
For Women, 2018
recipient for the second time
For Diversity, 2018
#40 out of 100 companies — recipient for the second consecutive year
For Giving Back, 2018
#22 out of 50 companies — recipient for the third consecutive year
For Manufacturing and Production, 2018
#1 out of 20 companies — recipient for the third time
Best Workplaces
International
#2 for Australia, 2018
recipient for the fifth consecutive year
Brazil, 2018
recipient for the fifth consecutive year
Canada, 2018
recipient for the ninth consecutive year
France, 2018
recipient for the first time
Netherlands, 2018
recipient for the first time
Mexico, 2018
recipient for the fifth time
Spain, 2018
recipient for the third time
FORTUNE 500 Largest U.S.-Based Companies
#240 in 2018
joined the list in 2003 — recipient for the 16th consecutive year
Top Employer Deutschland (Germany)
2019 award
recipient for the third consecutive year
FORTUNE 100 Best Companies to Work For (U.S.)
#11 in 2018
recipient for the ninth consecutive year
Download the 2018 Annual Review PDF
DOWNLOAD THE PDF
