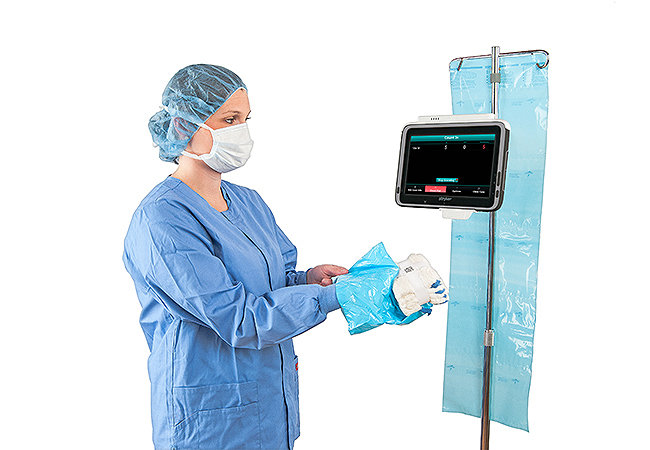
Surgicount360 Software
Document your surgical sponge count.
Fight to end retained surgical sponges.
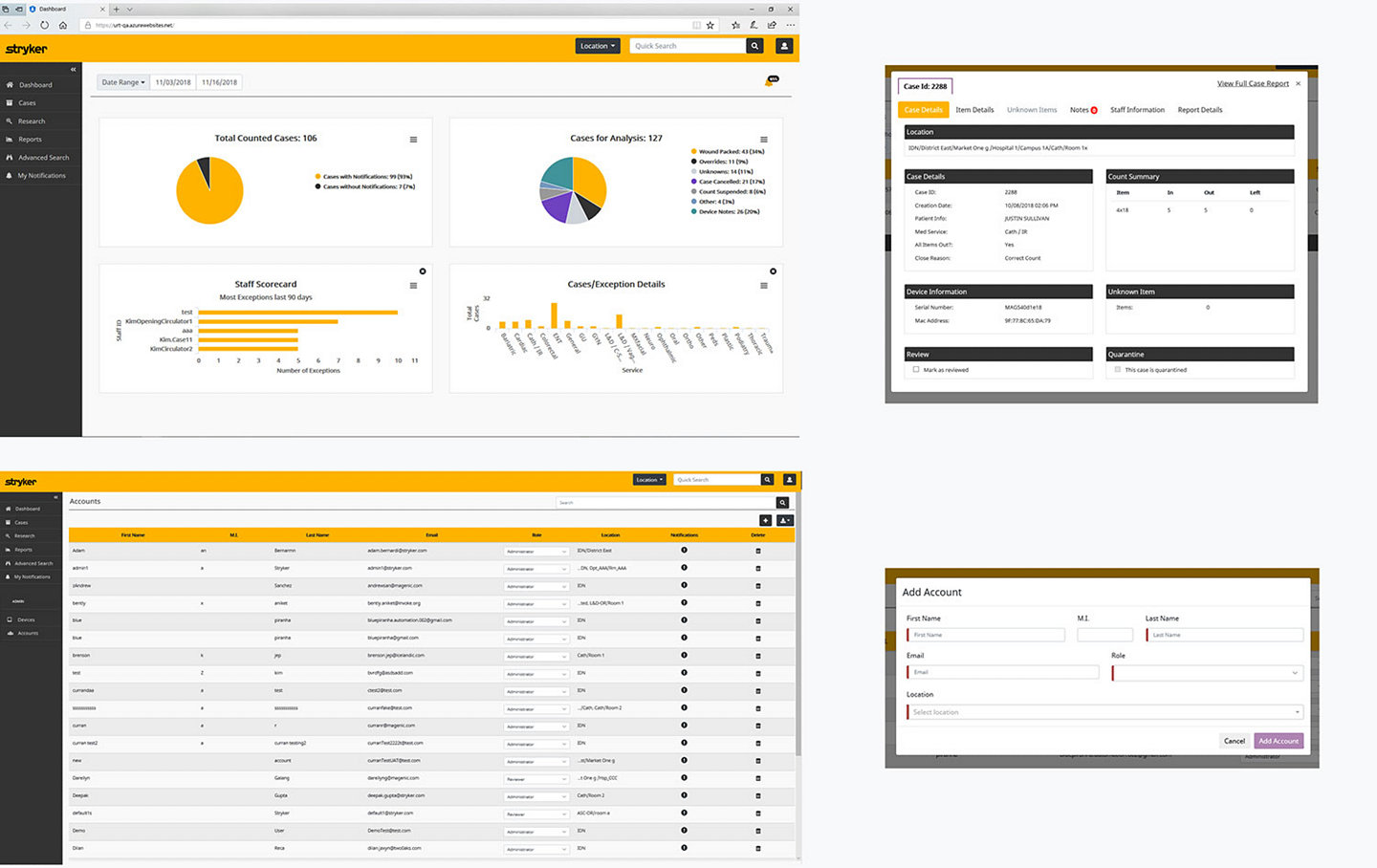
Why settle for a simple confirmation or “scan complete” message when we provide virtually unlimited reports to validate and document your sponge counts. Customize reports by procedure, service line, procedural room and staff – or aggregated data for your hospital, system or IDN. Whether micro or macro, you’ll have permanent, evidence-based data to support your internal needs or third-party audits.

Robust. Customizable. Actionable.
- Provides post-op accounting of each uniquely-identified sponge
- Demonstrates compliance to hospital policy or third-party best practices
- Supplies evidence-based data for external audits
- Generates actionable data for quality initiatives, outcomes percentages, issues resolution or performance optimization
- Captures deviations with justifications noted (e.g. wound pack)
- Yields objective metrics for inventory, purchasing, budget and staff planning
- Enables virtually endless report customization
Procedure-specific data capture
- Case data downloads into the aggregated SurgiCount360 Software database
- Exact time each unique Safety-Sponge was accounted for before and after removal from patient
- Type of procedure, procedure length and procedure sponge usage
- Staff changes during procedure (e.g. who exited, who entered and times)
- Sponge discrepancies and reason (e.g. wound pack, case cancellation)
- Surgical staff
- Time of day
- Patient ID
Medical errors are the third leading cause of death after heart disease and cancer, according to a 2016 study published in the fourth most cited medical journal in the world.14
“SurgiCount360 reporting provides a multitude of metrics – including by procedure, product, people or facility – so risky areas can be addressed and strengths can be replicated.”
Dylan Crotty, Vice President and General Manager

Accounting for the status of each and every unique sponge.

Radiopaque and each uniquely-coded with a fluid-wicking tag.
9100-003-595 Rev None
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: SAFE-T Lap, Safety-Sponge, Stryker, SurgiCount, SurgiCount360, and SurgiCounter. All other trademarks are trademarks of their respective owners or holders.
9100-003-611 Rev None