Understanding InSpace
The solution you've been waiting for
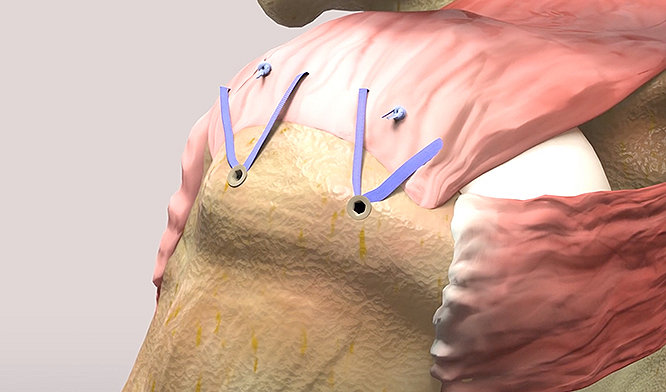
InSpace is the industry’s only minimally invasive biodegradable, subacromial balloon spacer for arthroscopic treatment of massive, irreparable rotator cuff tears (MIRCTs)†. It is designed to restore the subacromial space without requiring sutures or fixation devices.
The InSpace balloon implant helps fill an unmet clinical need and presents a streamlined, arthroscopic procedural option with potential for early functional recovery and pain relief for your patients.1
See the efficiency

Clinically proven results
InSpace is the only FDA-cleared MIRCT surgical solution supported by level 1 clinical evidence that preserves musculoskeletal tissues and bone and does not require the use of an anchor or permanent implant placement1.
InSpace InSights | Strengths of pivotal study featuring Dr. Nikhil Verma*
"Current strategies treating massive irreparable rotator cuff tears often present a challenge to surgeons and may require long and frustrating rehabilitation processes for patients,” said the lead investigator in the clinical study, Dr. Nikhil Verma, M.D. “The results of the study demonstrate the InSpace balloon is a ‘game-changer’ and presents a shorter, less invasive option that may enable sustained, clinically meaningful improvements in shoulder function and symptoms.”
—Nikhil N. Verma, M.D.
Shorter operative time
Early functional recovery
Early pain relief post procedure
Demonstrated safety profile
- InSpace resulted in a significant advantage for operative time (44 min) compared to partial repair (71min). (p<0.0001)
Figure 1. The percentage of subjects meeting the ASES score thresholds for the InSpace group (n = 93) and the partial repair group (n = 91). Significant improvements in the ASES score from baseline were noted in both groups at Month 12 and were maintained at Month 24.
- Early recovery at Week 6 was demonstrated in the InSpace group, as characterized by improvement in the ASES (figure 1), ROM (figure 2) and Constant (figure 3) scores:
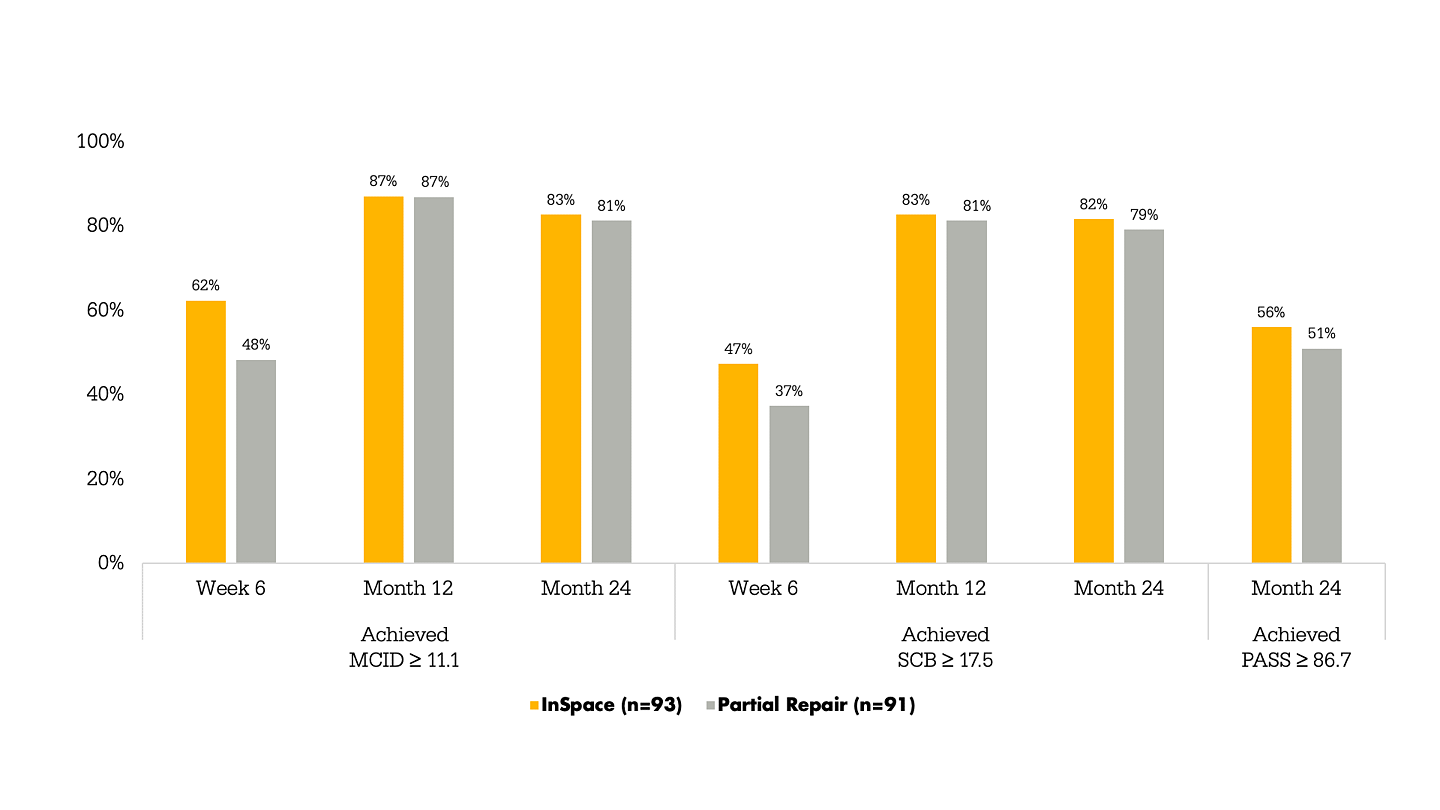
Figure 1. Percentage of subjects meeting the American Shoulder and Elbow Score (ASES) minimally clinically important difference (MCID), substantial clinical benefit (SCB) and patient acceptable symptomatic state (PASS) thresholds for the InSpace group (n=93) and the partial repair group (n=91). Significant improvements in the ASES score from baseline were noted in both groups at Month 12 and were maintained at Month 24.
ROM change from baseline through Month 24
Month 24 intent-to-treat population
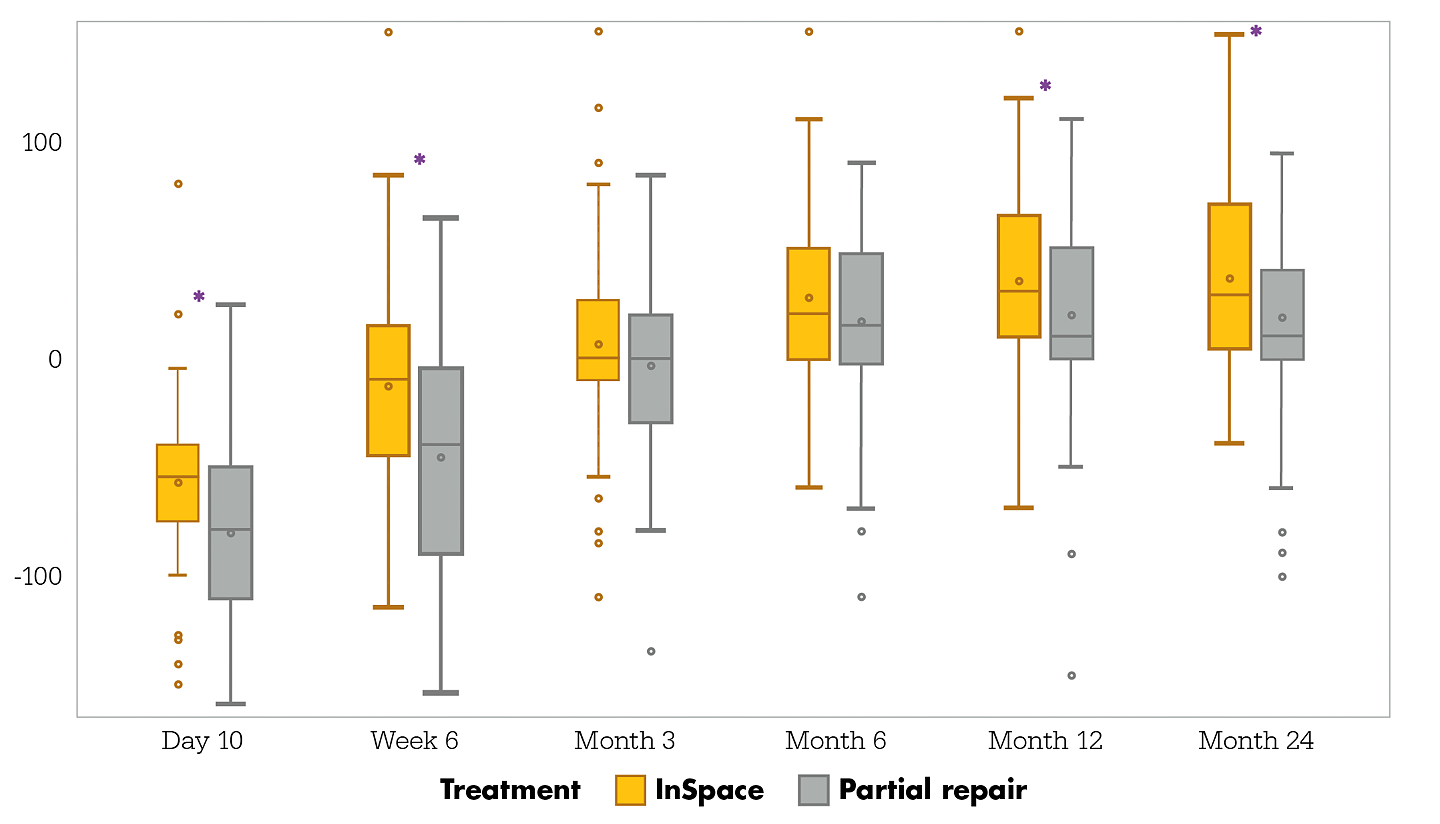
Figure 2. Box-and-whisker plot showing forward elevation Range of Motion (ROM), presented as a change from baseline, for the InSpace group (n=93) and the partial repair group (n=91). ROM can range from 0° to 180°, with a higher value indicating improvement. Significant improvements, indicated by asterisks, from baseline in favor of the InSpace group were noted at Day 10 (p=0.041), Week 6 (p=0.0001), Month 12 (p=0.0048) and Month 24 (p=0.003). The median values are indicated with horizontal lines, IQRs are indicated with boxes, and whiskers denote data
points within ± 1.5 IQR. Circles indicate outliers.
Constant-Murley change from baseline through Month 24
Month 24 intent-to-treat population
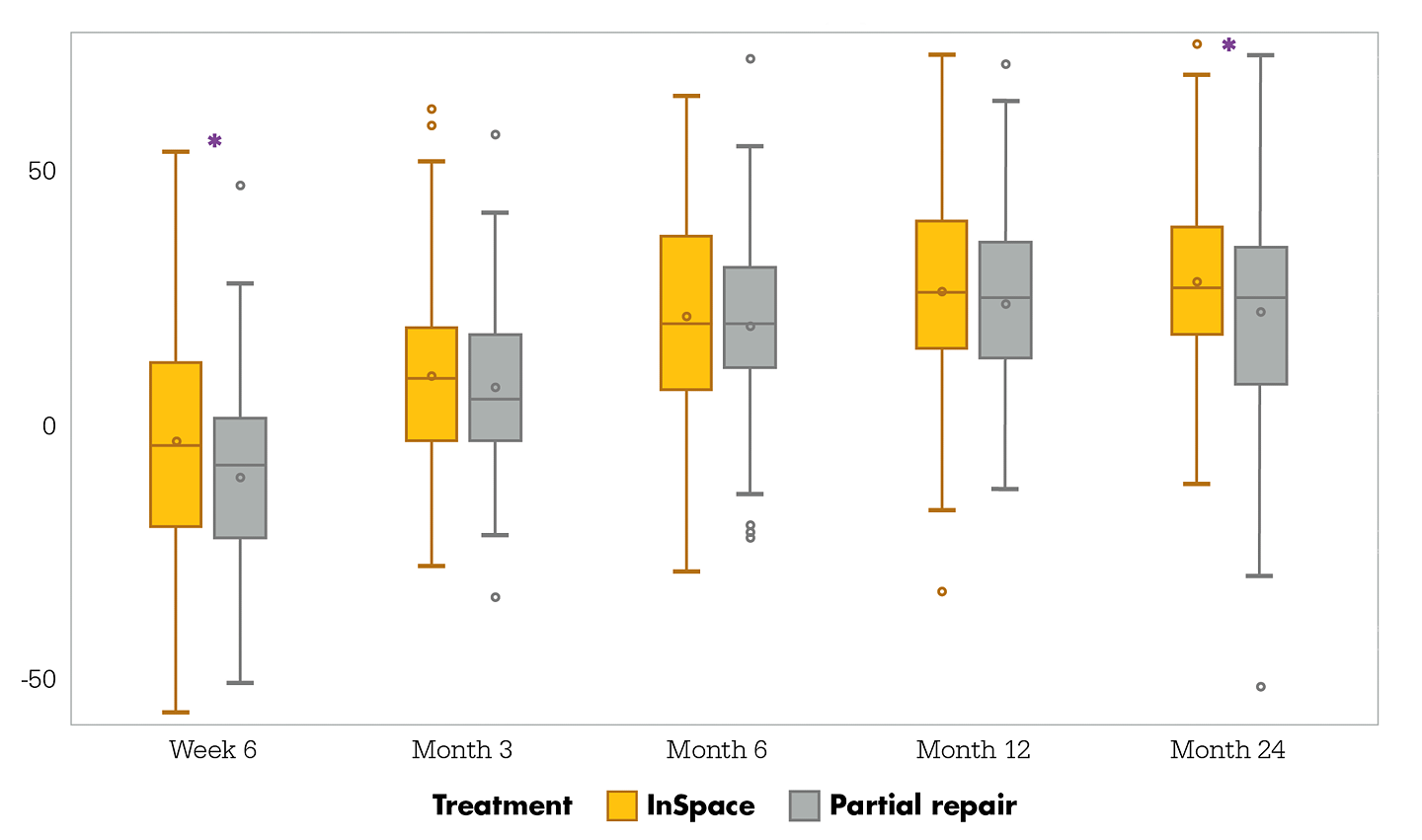
Figure 3. Box-and-whisker plot showing the overall Constant- Murley Score, presented as a change from baseline, for the InSpace group (n=93) and the partial repair group (n=91). The Constant score can range from 0 to 100, with a higher score indicating improvement. Significant improvements, indicated by asterisks, from baseline in favor of the InSpace group were noted at Week 6 (p=0.021) and Month 24 (p=0.05). The median values are indicated with horizontal lines, IQRs are indicated with boxes, and whiskers denote data points within ± 1.5 IQR. Circles indicate outliers.
- No subject experienced a Serious Adverse Device Effect (SADE) through the entire 24 months of study.
- No InSpace devices required explanation.
- No adverse device effects.
- Similar rates of Subsequent Secondary Surgical Intervention (SSSI) through 24 months (InSpace: 4; Partial Repair: 3).
- InSpace demonstrated comparable results out to two years compared to PR.

Pivotal study patient selection
Inclusion
- Full thickness MRCT
- Measuring ≥ 5 cm in diameter
- Involving ≥ 2 tendons
- VAS score >30
- 4 months failed conservative Rx
- Functional deltoid muscle with preserved passive ROM
- Good health
For complete eligibility criteria, please refer to the JBJS publication.
Exclusion
- Known allergy to InSpaceTM implant
- Severe glenohumeral or acromiohumeral arthritis
- Full thickness cartilage loss on MRI
- Deltoid defect or palsy
- Partial thickness tears of the supraspinatus
- Glenohumeral joint trauma, infection or necrosis
- Fully reparable cuff tear
- Requires concomitant subscapularis or labral repair
Surgeon experience case presentations

Surgical technique

Post-op and rehab

Upcoming events

Economics and reimbursement
For assistance with your reimbursement questions, please contact Stryker's Reimbursement Services at 800 698 9985 (option 1) or inspace.reimbursement@stryker.com.

Patient testimonials
1. Verma N, Srikumaran U, Roden CM, Rogusky EJ, Lapner P, Neill H, Abboud JA. (2022). InSpace implant compared with partial repair for treatment of full-thickness massive rotator cuff tears. J Bone Joint Surg Am. Advance online publication. doi. 10.2106/JBJS.21.00667. http://ow.ly/wjSB50IRYym
*A Stryker consultant
† The InSpace™ subacromial tissue spacer system is indicated for the treatment of patients with massive, irreparable full-thickness torn rotator cuff tendons due to trauma or degradation with mild to moderate gleno-humeral osteoarthritis in patients greater than or equal to 65 years of age whose clinical conditions would benefit from treatment with a shorter surgical time compared to partial rotator cuff repair.
1000903848 Rev. B


























?wid=319&hei=319&fmt=png&fit=constrain,true)