Citrelock®
Tendon Fixation Device System
The future of bioresorbables
Citrelock Tendon Fixation Device System is a biotenodesis screw used for tendon fixation in foot and ankle procedures, featuring Citregen.
Citrate, calcium, and phosphate are released to the surrounding tissue.
Polymer resorption continues as the bioceramic becomes metabolized.
The biomimetic implant is replaced by the normal tissue anatomy.
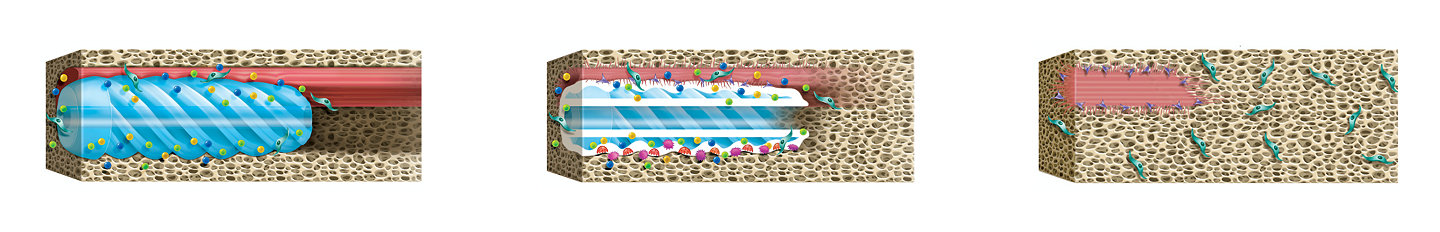
Citregen® material technology
Citregen is a biomaterial designed to mimic the hydroxyapatite content of native bone.2 It is a resorbable biocomposite with a controllable biodegradation process with no chronic inflammation.1,3,* Citregen slowly releases citrate, calcium, and phosphate, which play a significant role in bone regeneration.6
Citregen vs. thermoplastics
- A sheep study at Colorado State University showed evidence of biocompatibility and biologic growth into porous Citregen implants, absent of any signs of chronic inflammation.7
- Citregen elutes into surrounding tissue via surface erosion, not bulk degradation.1
- Citregen’s crosslinked structure provides elasticity and demonstrates recovery from compressive loads that would cause thermoplastics to permanently deform. Even with a high bioceramic content, Citregen composites retain their toughness withstanding large mechanical loads and strain.6

Tendon integrity
- Designed to not lacerate the tendon during insertion.5
- Improved pull-out strength compared to thermoplastic materials.2
- Compressive strength similar to cortical bone.4
Citregen key points
- Elastomeric – Elastomeric properties provide strong pull-out strength in comparison to thermoplastic polymers or composites1,4,*
- Resorbable - Citrate, calcium, and phosphate molecules start releasing into the system on day one6
- No bulk degradation, which means no chronic inflammation as the implant resorbs with controlled predictability7,*

Implant selection
- Designed to cover a breadth of foot & ankle procedures
- Sterile-packed, individual sizes
Nine strategic sizes
- 4mm × 10mm
- 5mm × 10mm
- 5mm × 15mm
- 6mm × 15mm
- 6mm × 23mm
- 7mm × 15mm
- 7mm × 23mm
- 8mm × 15mm
- 8mm × 23mm
Contact us
Send us a message to learn more about Citrelock
Have questions or inquiries? We’re here to help! Fill out the contact form, and our dedicated team will get back to you promptly. Your feedback and inquiries are important to us, and we look forward to assisting you with any information or support you may need.
Are you a patient looking for more information or a physician in your area?
Click the button below for our patient website.
References
- FDA 510(k) K200725
- TM-210503, Acuitive Technologies, Inc
- Yang J, Webb AR, et al. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27(9):1889-98. doi: 10.1016/j.biomaterials.2005.05.106.
- TM-190301, Acuitive Technologies, Inc.
- TM-210202, Acuitive Technologies, Inc.
- Wang H, Huddleston S, et al. Enabling Proregenerative Medical Devices via Citrate-Based Biomaterials: Transitioning from Inert to Regenerative Biomaterials. Adv Mater; 2024:36(6):17. doi: 10.1002/adma.202306326
- Acuitive Technologies, Inc. website
*Studies in GLP animal bone models
CITE-AWI-2, 08-2024

.pdf.thumb.319.319.png)
.pdf.thumb.319.319.png)
.pdf.thumb.319.319.png)
