Stryker’s InSpace subacromial balloon spacer two-year, Level 1 randomized controlled clinical study published in the Journal of Bone and Joint Surgery
03-May-2022
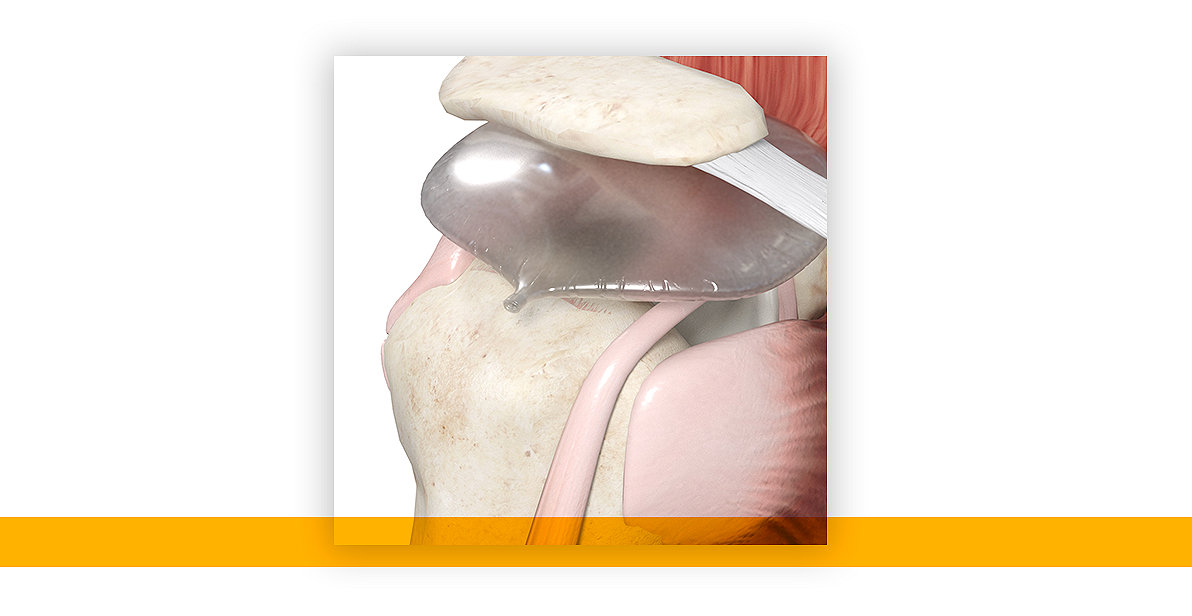
InSpace implant demonstrated to be a viable option for patients with massive irreparable rotator cuff tears.*
Kalamazoo, Michigan, USA – Stryker (NYSE:SYK) announced today the publication of results from a multicenter, single-blinded, randomized controlled trial comparing Stryker’s InSpace implant with partial repair for the treatment of full-thickness massive rotator cuff tears (MIRCTs) in the Journal of Bone and Joint Surgery (JBJS).
The comparative study evaluated the efficacy and safety of Stryker’s InSpace implant with arthroscopic partial repair in patients with irreparable, posterosuperior MIRCTs.
Results of the two-year study demonstrated the InSpace implant as an appropriate alternative to partial repair in patients with MIRCTs. The study also revealed notable patient benefits including early functional recovery and pain relief combined with a shorter operative time.
MIRCTs are one of the most common causes of shoulder dysfunction.1,2,3 The InSpace balloon implant* is a breakthrough solution in the shoulder continuum of care that addresses MIRCTs and provides a new, simple surgical treatment option, allowing surgeons to better meet the needs of their patients.
“We are excited to see the dedicated research team’s hard work, robust patient follow-up and high-quality data from the U.S. InSpace trial accepted and published by one of the most prestigious orthopaedic journals,” said the lead investigator in the clinical study, Nikhil Verma, M.D. “Improving more patients’ lives remains our top priority. These results demonstrating InSpace as a safe and effective option with earlier functional recovery, pain relief and shorter operative times are helping us to change the game in shoulder care and creating better care for patients.”
About the clinical study
The purpose of the Level 1 InSpace pivotal study – a prospective, single-blinded, multi-center, randomized, controlled trial was to assess the safety and effectiveness of the InSpace Implant (IS) compared to Partial Repair (PR). The study evaluated 184 (n=93 IS; n=91 PR) randomized eligible patients with symptomatic massive full thickness rotator cuff tears that failed non-operative management, through 24 months of follow-up.1
Click here to read the full JBJS article on the study.
About the balloon
InSpace is a biodegradable implant designed to restore the subacromial space, providing a less invasive solution compared to other surgical treatment options that require fixation devices or grafts and has been demonstrated to improve shoulder motion and function.4
More information about the InSpace balloon implant is available at www.stryker.com/inspace.
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Medical and Surgical, Neurotechnology, Orthopaedics and Spine that help improve patient and healthcare outcomes. Alongside its customers around the world, Stryker impacts more than 100 million patients annually. More information is available at www.stryker.com.
Media contact
Kara Rasmussen
Sr. Director, Communications and PR
kara.rasmussen@stryker.com
Phone: 408 529 7512
- Novi M, et al. Irreparable rotator cuff tears: challenges and solutions. Orthop Res Rev. 2018; 10:93-103.
- Yamamoto A, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010; 19(1):116-120.
- Minagawa H, et al. Prevalence of symptomatic and asymptomatic rotator cuff tears in the general population: From mass-screening in one village. J Orthop. 2013; 10(1):8-12.
- Verma N, Srikumaran U, Roden CM, Rogusky EJ, Lapner P, Neill H, Abboud JA. (2022). InSpace implant compared with partial repair for treatment of full-thickness massive rotator cuff tears. J Bone Joint Surg Am. Advance online publication. doi. 10.2106/JBJS.21.00667
