Neuroform EZ
Stent System
_____________________________________
Flexible design. Enhanced delivery.
The Neuroform EZ Stent System is comprised of a self-expanding Nitinol stent, pre-loaded on an enhanced Stent Delivery Wire. The Neuroform Stent is engineered for conformability, flexibility, and accessibility.

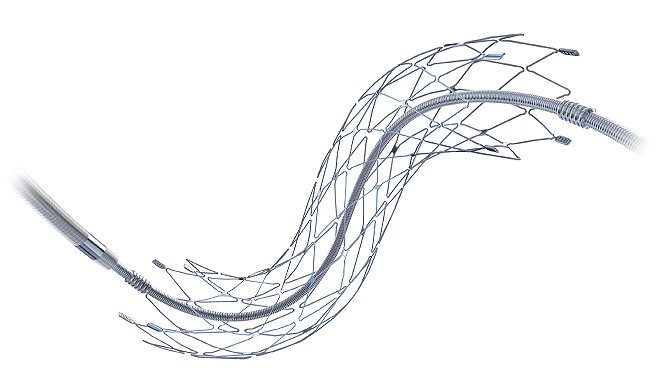
The Neuroform EZ Stent System is under a Humanitarian Device Exemption (IDE). IRB is required prior to use.
AP001666 v2.0
