03-Aug-2020
Kalamazoo, Michigan, USA – August 3, 2020 – Stryker announced today that it has received U.S. Food and Drug Administration (FDA) approval for an expanded indication of its Neuroform Atlas Stent System, becoming the first and only adjunctive stent approved for use in the posterior (back of the brain) circulation. Aneurysms in the posterior circulation rupture more frequently and are generally more difficult to treat. With the approval of the Neuroform Atlas adjunctive stent for the posterior circulation, long-term treatment is more feasible.
Already approved for use in the anterior circulation, the expanded indication was granted based on robust clinical trial evidence proving the safety and efficacy of the device. The combined patients from both the anterior and posterior cohorts totaled 298 patients, making it the largest study of its kind.
“The Atlas Posterior PMA trial data shows a compelling advancement in the treatment of wide-neck posterior aneurysms,” said Dr. Sam Zaidat, Director of the Neuroscience and Stroke Center at Mercy Health System in Toledo, Ohio, and Co-Principal Investigator of the U.S. Neuroform Atlas investigational trial. “Posterior stent-assisted coiling with Neuroform Atlas achieved an impressive high rate of complete occlusion in this very challenging location at 76.7%. Equally impressive was the 4.3% primary safety rate.”
An estimated 6.5 million people in the United States have unruptured brain aneurysms with an average rupture rate of 1 every 18 minutes. Ruptured brain aneurysms are fatal in roughly 50% of cases.1
"Complete occlusion, or complete blocking of blood flow, is the gold standard in determining long-term aneurysm healing," said Dr. Brian Jankowitz, Director, Cerebrovascular Surgery at the Cooper Neurological Institute in Camden, New Jersey. "With the additional challenges that come with treating posterior circulation aneurysms, we never would have anticipated reaching occlusion rates that rival those found in the anterior circulation. Now with Neuroform Atlas, those same high rates are achievable."
“The results from the Atlas Posterior PMA trial demonstrate that physicians can now address the more difficult posterior circulation aneurysms, offering hope for better outcomes in that patient population,” said Mark Paul, president of Stryker’s Neurovascular division. “This expanded indication of Neuroform Atlas, as the first and only adjunctive stent for use in the posterior circulation, reflects our ongoing commitment to advancing stroke care for patients with cerebrovascular disease.”
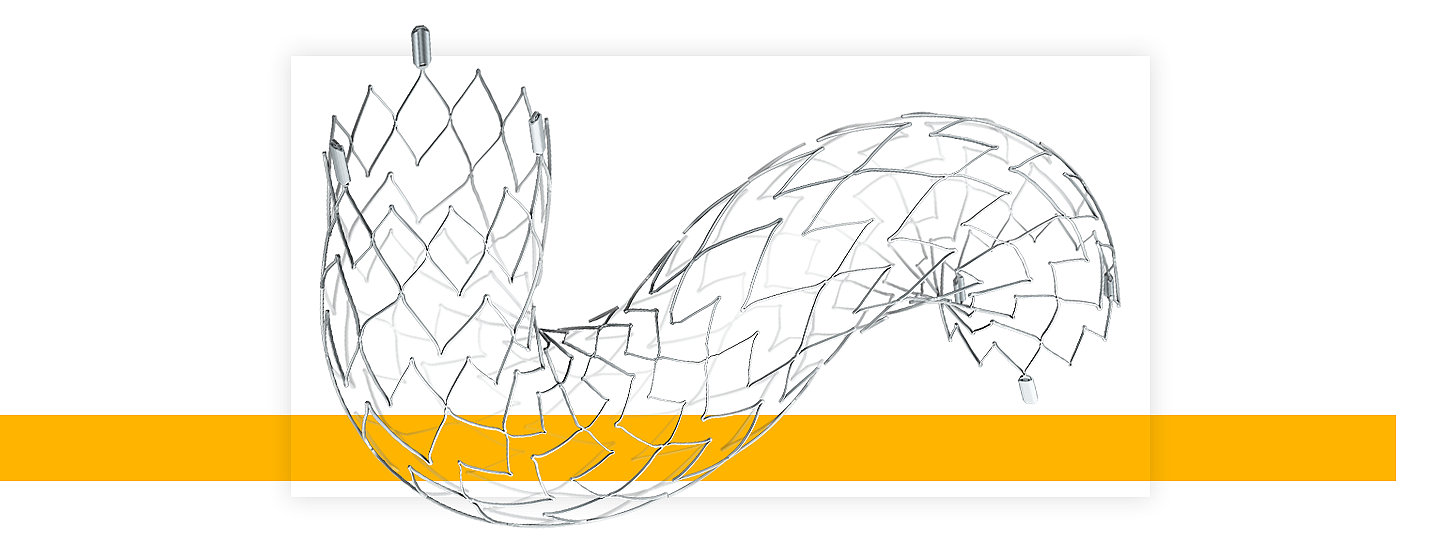
About Neuroform Atlas Stent System
Stryker’s Neuroform Atlas Stent System is a self-expanding nitinol stent used in conjunction with metal coils to pack weakened blood vessel sacs called aneurysms within the brain. The stent is positioned across the aneurysm neck to hold metal coils in place and occlude the aneurysm.
The Neuroform Atlas Stent System is indicated for use with neurovascular embolization coils in the anterior and posterior circulation of the neurovasculature for the endovascular treatment of patients ≥ 18 years of age with saccular wide-necked (neck width ≥ 4 mm or a dome-to-neck ratio of < 2) intracranial aneurysms arising from a parent vessel with a diameter of ≥ 2.0 mm and ≤ 4.5 mm.
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Orthopaedics, Medical and Surgical, and Neurotechnology and Spine that help improve patient and hospital outcomes. More information is available at www.stryker.com.
Media contact
Keri Laden
Global Communications Manager
keri.laden@stryker.com
1. Brain Aneurysm Foundation Statistics. www.bafound.org
