LIFEPAK 1000 defibrillator
LIFEPAK 1000 defibrillator
Simple. Flexible. Powerful.
Not every cardiac emergency is the same. Neither is every responder. Your world demands flexibility—and that’s exactly what the LIFEPAK 1000 defibrillator from Stryker delivers.

Simple. Flexible. Powerful.
If you’re first on the scene of a cardiac emergency, the LIFEPAK 1000 AED can help improve lifesaving outcomes and speed the transition of patients to the next critical level of care.

Product highlights
LIFEPAK 1000In a police and fire rescue vehicle, or out in the field, you’re always ready to respond to cardiac emergencies. The LIFEPAK 1000 provides powerful defibrillation, long battery life, and durability that can stand up to severe environments.

Durable, vehicle-friendly construction
- Endures rigorous drop-tests and includes protective case and bumpers for durability in the harshest environments.
- Designed to ride along in vehicle without damage from continuous vibrations and other movement.

Powerful and easy to use
- Loud voice prompts and on-screen graphics provide clear guidance on applying electrodes and initiating a shock to ensure user confidence.
- Escalating energy to 360 J, biphasic, for difficult to defibrillate patients.
- Provides battery power for up to 220 shocks – escalating to 360 Joules – delivering 17 hours of monitoring time.

Clinically advanced, with robust features
- Exclusive cprMAX™ Technology minimizes CPR interruptions by allowing responders to perform pre-shock CPR while the AED charges.
- Features including a CPR countdown timer, ECG capability, a shock counter, and programmable settings – all on a large, easy-to-read LCD screen.
Compatibility and connectivity
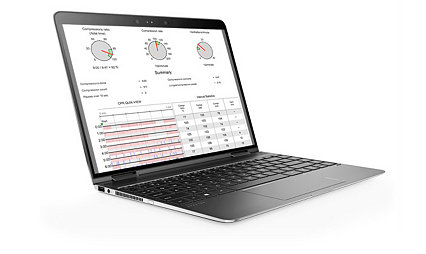
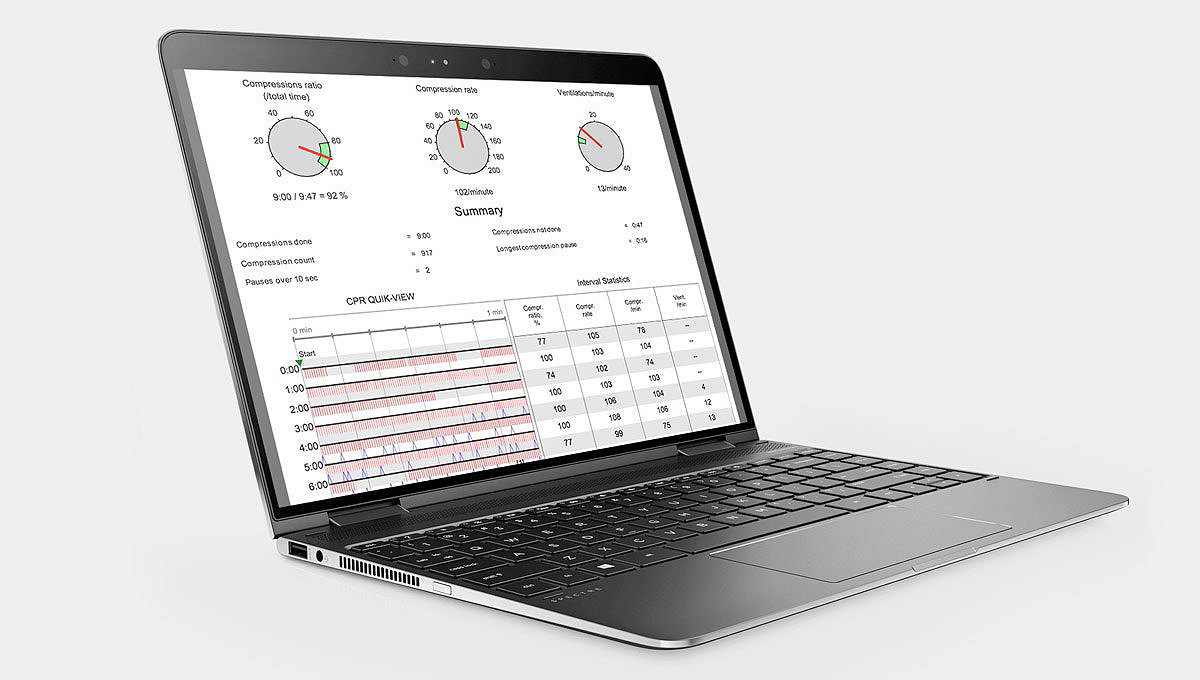
Compatibility with other LIFEPAK electrodes and monitors speeds the transition to the next level of care. And, post-event patient and performance data can be easily viewed, analyzed and managed with CODE-STAT data review software – helping to improve future response and target training needs.
Take the next step
Want to talk to your Sales Representative? Please complete the form below and we’ll connect you with your local representative.
When you invest in our lifesaving devices and data products, you are buying more than quality design and manufacturing—you’re also buying our unrivaled commitment to customer service and support.
Technical support
We offer one of the largest networks of technical service representatives in the industry. We’ll work with you to quickly assess any issues and find the best solution.
Training and education
As your lifesaving partner, Stryker offers training support, seminars, podcasts, and access to clinical research on the latest resuscitation strategies and tips.
Related products
LIFEPAK CR2 defibrillator
In a cardiac emergency, the LIFEPAK CR2 defibrillator connects everything and everyone involved in the response – reducing delays in treatment and improving care across the lifesaving system.
Learn moreHeartSine samaritan PAD 350P/360P
The compact and easy-to-use HeartSine AEDs empower first responders with lifesaving technology, while integrated Wi-Fi connectivity ensures the devices are ready when needed most.
Learn moreCODE-STAT data review software
Turn your passion for saving lives into targeted improvements — CODE-STAT data review software and service lets you easily understand team performance immediately after response.
Learn moreThis document is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it.
The information presented is intended to demonstrate Stryker’s product offerings. A healthcare professional must always refer to operating instructions for complete directions for use indications, contraindications, warnings, cautions, and potential adverse events, before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your representative if you have questions about the availability of Stryker’s products in your area. Specifications subject to change without notice. The products depicted are CE marked in accordance with applicable EU Regulations and Directives.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: LIFEPAK, LIFENET, LUCAS, HealthEMS, RevNet, CODE-STAT, Physio-Control, HeartSine, samaritan, LIFEPAK CR, TrueCPR, REDI-CHARGE, QUIK-COMBO, EDGE, cprINSIGHT, ClearVoice, QUIK-STEP, LIFEPAK TOUGH, HomeSolutions.net, RELI, REDI-PAK, Shock Advisory System, LIFELINKcentral. All other trademarks are trademarks of their respective owners or holder. The yellow and black color scheme is a registered trademark of Stryker Corporation.
LIFEPAK devices: CE Class IIb (0123)
HeartSine device: CE Class IIb (0123)
LUCAS device: CE Class IIb (2460)
EC REP: Stryker European Operations Limited | Anngrove, IDA Business & Technology Park | Carrigtwohill, Co. Cork, T45 HX08 | Ireland
M0000007484 REV AA