LIFEPAK CR2 defibrillator
LIFEPAK CR2 defibrillator
Connected. Ready.
In a cardiac emergency, the LIFEPAK CR2 defibrillator connects everything and everyone involved in the response – reducing delays in treatment and improving care across the lifesaving system.

Who will be saved next?
When you choose the LIFEPAK CR2 defibrillator, you’re choosing a connected solution that empowers bystanders to help save a life – all while backed by innovative technology that allows continuous CPR and streamlines communication across the system of care.
With a design influenced by user feedback, the LIFEPAK CR2 is at the heart of a complete AED response system.

Product highlights
LIFEPAK CR2The LIFEPAK CR2 is a powerful, easy-to-use AED1. Advanced technology including cprINSIGHT, Wi-Fi and cellular connectivity and continuous self-monitoring ensure devices and responders are always prepared to deliver swift, lifesaving treatment.

Self-monitoring AED program
Battery not charged? AED not where it’s supposed to be? The LIFELINKcentral AED program manager monitors each LIFEPAK CR2 connected to a cellular or Wi-Fi network and alerts you to anything that may affect device readiness—all automatically. This greatly reduces the effort and expense of managing your AED program, while increasing your program’s readiness and effectiveness.
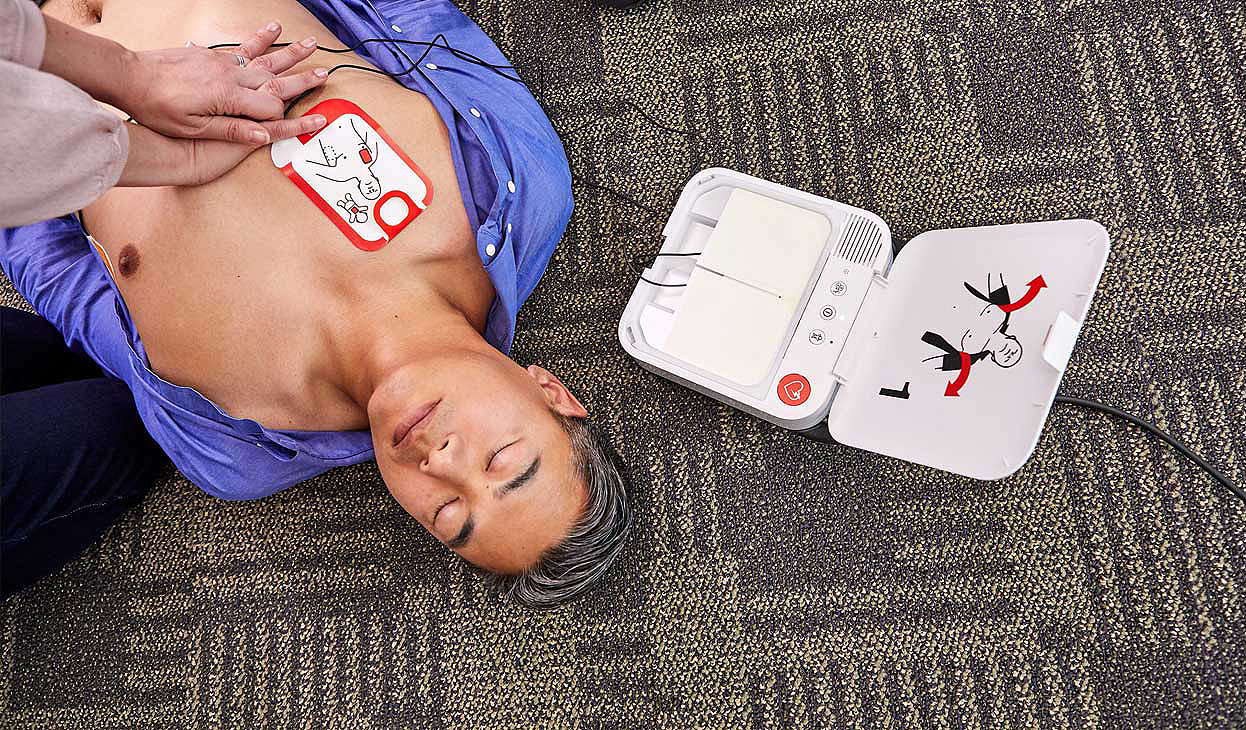
Continuous CPR
More CPR means improved blood circulation and better odds of survival2-3. The LIFEPAK CR2 allows chest compressions during ECG rhythm analysis, reducing pauses between CPR and defibrillation. Once CPR begins, cprINSIGHT technology – unique to only LIFEPAK CR2 – automatically analyzes and detects if a shock is needed. This significantly reduces pauses in chest compressions, even eliminating pauses if the rhythm is determined to be non-shockable.
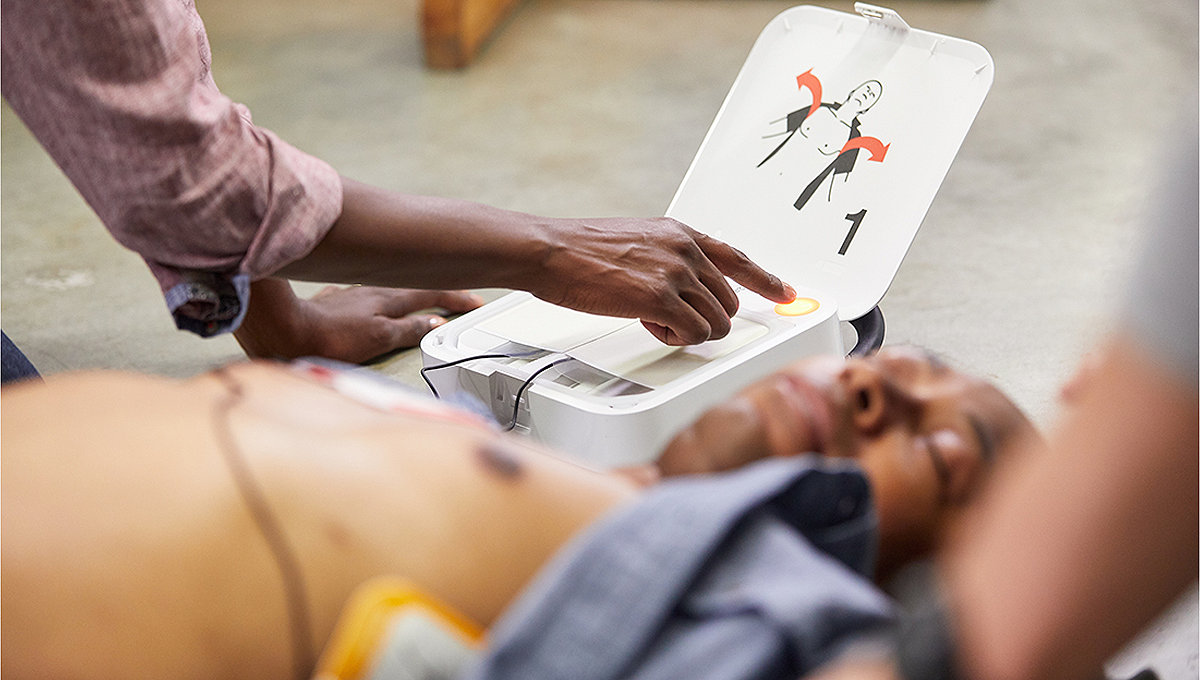
Connected to responders
The right information at the right time can make a lifesaving difference. With the LIFEPAK CR2, Wi-Fi connectivity gives emergency responders a complete picture of each cardiac arrest event. So even before they arrive, emergency professionals are better prepared and understand the details of the shocks given, see the patient’s actual ECG, and more.
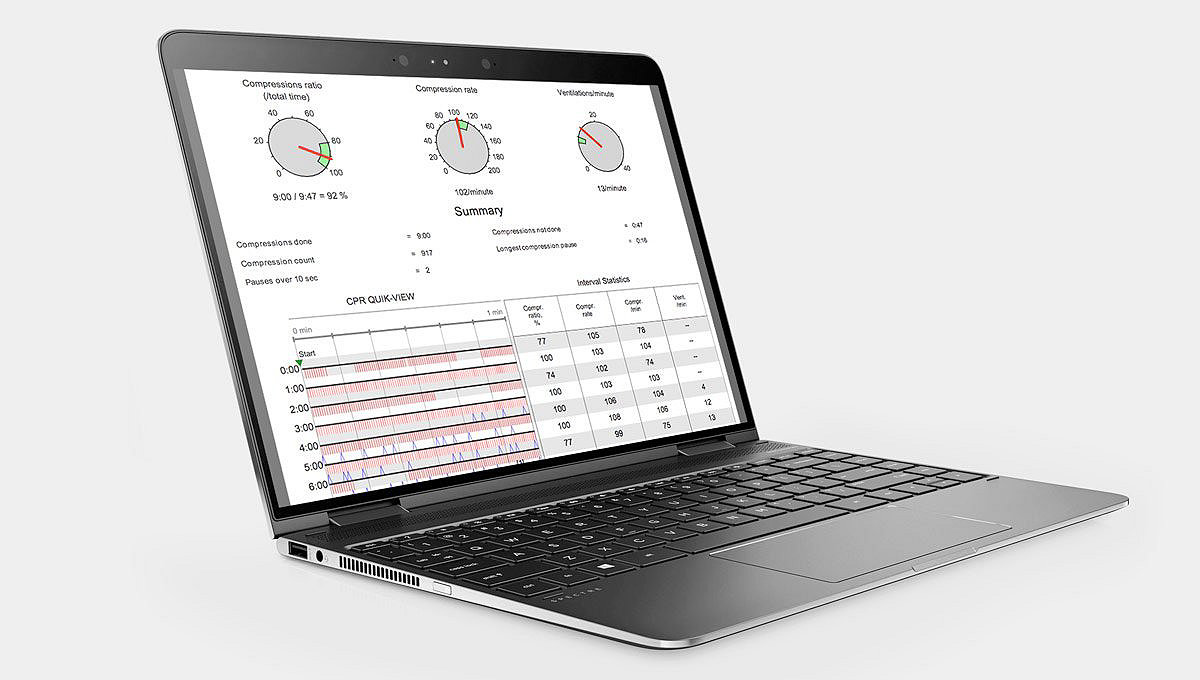
Continuity of care
The LIFEPAK CR2 streamlines continuity of care by seamlessly sending all AED information to the LIFENET System, helping to speed the transition to the next level of care with emergency services and the hospital.

Simple operation
The LIFEPAK CR2 uses simple graphics, audible instructions and automated features to help users remain focused1. A child mode button provides reduced energy and CPR guidance specifically for children.
Program Management
Management solutions tailored to your needs
Our AED program management options are supported by trained AED experts who are just a quick phone call or email away. We offer complete solutions from start-up to ongoing management, with the flexibility to customize for your environment and resources.
Take the next step
Want to talk to your Sales Representative? Please complete the form below and we’ll connect you with your local representative.
When you invest in our lifesaving devices and data products, you are buying more than quality design and manufacturing—you’re also buying our unrivaled commitment to customer service and support.
Technical support
We offer one of the largest networks of technical service representatives in the industry. We’ll work with you to quickly assess any issues and find the best solution.
Training and education
As your lifesaving partner, Stryker offers training support, seminars, podcasts, and access to clinical research on the latest resuscitation strategies and tips.
Related products
LIFEPAK 1000 defibrillator
Not every cardiac emergency is the same. Neither is every responder. Your world demands flexibility—and that’s exactly what the LIFEPAK 1000 defibrillator from Stryker delivers.
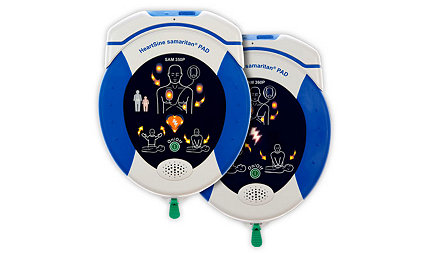
Learn moreHeartSine samaritan PAD 350P/360P
The compact and easy-to-use HeartSine AEDs empower first responders with lifesaving technology, while integrated Wi-Fi connectivity ensures the devices are ready when needed most.
Learn moreHeartSine samaritan PAD 500P
When every second counts, you need an AED that you can count on. Integrated Wi-Fi connectivity ensures the compact HeartSine samaritan AED will be ready when it’s needed. In addition to delivering a lifesaving shock, the device also provides real-time feedback to help the rescuer perform effective CPR.
Learn moreThis document is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it.
The information presented is intended to demonstrate Stryker’s product offerings. A healthcare professional must always refer to operating instructions for complete directions for use indications, contraindications, warnings, cautions, and potential adverse events, before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your representative if you have questions about the availability of Stryker’s products in your area. Specifications subject to change without notice. The products depicted are CE marked in accordance with applicable EU Regulations and Directives.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: LIFEPAK, LIFENET, LUCAS, HealthEMS, RevNet, CODE-STAT, Physio-Control, HeartSine, samaritan, LIFEPAK CR, TrueCPR, REDI-CHARGE, QUIK-COMBO, EDGE, cprINSIGHT, ClearVoice, QUIK-STEP, LIFEPAK TOUGH, HomeSolutions.net, RELI, REDI-PAK, Shock Advisory System, LIFELINKcentral. All other trademarks are trademarks of their respective owners or holder. The yellow and black color scheme is a registered trademark of Stryker Corporation.
LIFEPAK devices: CE Class IIb (0123)
HeartSine device: CE Class IIb (0123)
LUCAS device: CE Class IIb (2460)
EC REP: Stryker European Operations Limited | Anngrove, IDA Business & Technology Park | Carrigtwohill, Co. Cork, T45 HX08 | Ireland
Sources
1 - Physio-Control Internal Semi-Automatic AED Comparison Usability Study, August 2016.
2 - Berg RA, Hemphill R, Abella BS, Et al. Part 5: Adult Basic Life Support: 2010 American Heart Association Guidelines for Cardiopulmonary
Resuscitation and Emergency Cardiovascular Care. Circulation. 2010:122[suppl 3]:S694.
3 - Brouwer T, Walker R, Chapman F, Koster, R. Association Between Chest Compression Interruptions and Clinical Outcomes of Ventricular
Fibrillation Out-of-Hospital Cardiac Arrest. Circulation. 2015;132:1030-1037.
M0000007484 REV AA