LIFEPAK 15 monitor/defibrillator
LIFEPAK 15 monitor/defibrillator
A device by professionals, for professionals
Rely on the LIFEPAK 15 monitor/defibrillator for the confidence you need in emergencies and the highest available escalating energy, up to 360 joules (360J).

Make high performance easier in challenging situations.
With six decades of serving EMS professionals, this LIFEPAK TOUGH tool helps skilled teams boost their performance and improve patient outcomes.
Proven clinical performance
Take advantage of escalating energy up to 360J—which has been shown to improve conversion rates for difficult-to-defibrillate patients.1-4 Elevate STEMI care with the University of Glasgow ECG Analysis Program and ST-segment trend monitoring, which continuously monitors all 12-leads, alerting you to changes.

Work efficiently
From intuitive controls to a dual-battery system and a large 21.3 cm anti-reflective color display that changes to high-contrast mode with a single button, the LIFEPAK 15 helps you work more efficiently so you can keep your focus on your patients.

LIFEPAK TOUGH durability
With more than a dozen durability enhancements from the previous generation of LIFEPAK devices, the LIFEPAK 15 is our toughest yet – built to withstand drops, shocks, and extreme vibration.

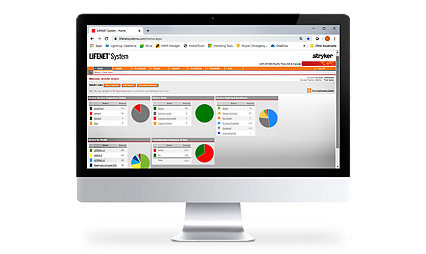
Continually improve team response and outcomes
The LIFENET System connects the LIFEPAK 15 through the cloud to transmit emergent patient data to the hospital while enabling ease of documentation, care team activation for time-sensitive emergencies, and valuable insights to support continuous performance improvement.

Streamline device management and maintenance
Help ensure the device is always ready to go with two reliable batteries and a daily self-test that alerts you to possible issues before you experience them in the field. Easily track device health and readiness via your LIFENET Asset management system.

Key features
On your side wherever the next call takes youYou can choose the LIFEPAK 15 for all the ways it helps you do your tough job even better.
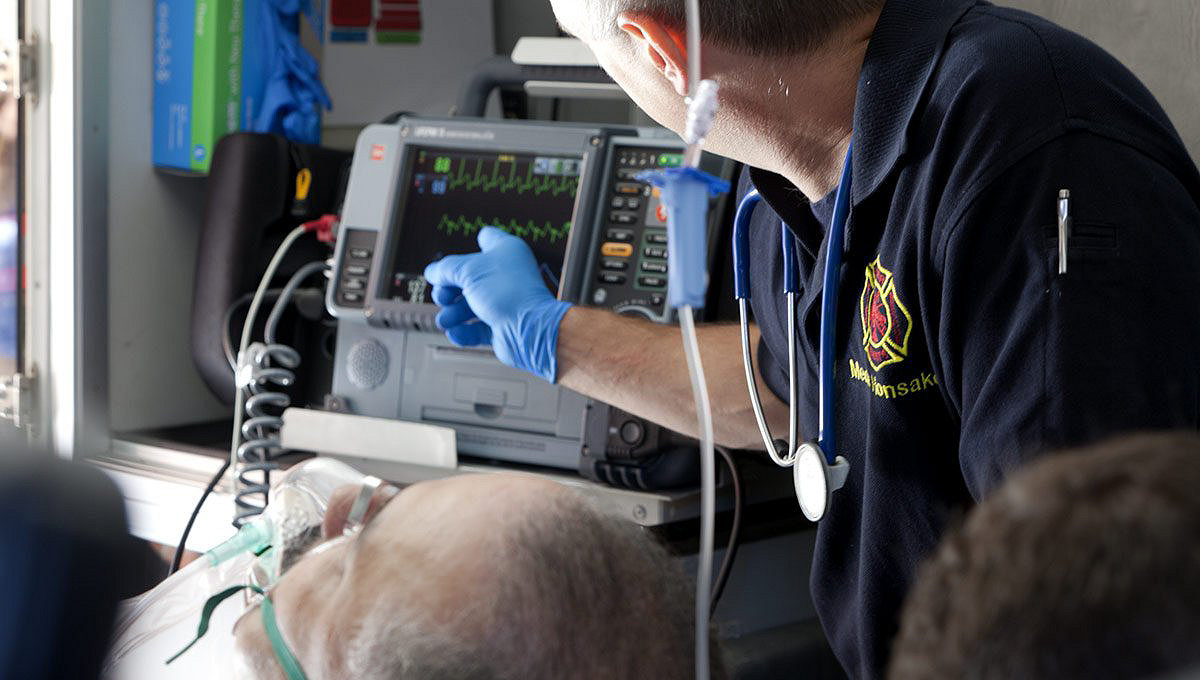
Easy to use so care teams can focus on patients
- Dual-mode display screen that provides maximum visibility
- All key functionality with a single button push
- Easy access to cables, connections and printer

Supports improved resuscitation results
- Proven CPR guidance—shown to guide responders to perform compressions at 100/minute and avoid over-ventilation*
- Capture all displayed waveforms and CPR quality for post-event review
- The highest escalating energy available, up to 360J biphasic, for difficult-to-defibrillate patients
*Kern KB, Stickney RE, Gallison L, Smith R. Metronome improves compression and ventilation rates during CPR on a manikin in a randomized trial. Resuscitation. 2010;81:206-210

Improves operational effectiveness
- Upgradeable platform to adapt to evolving protocols and new care guidelines
- Instant access to device health and readiness data through LIFENET Asset
- Enhances team review with rapid data export to CODE-STAT post-event review software for QA/QI
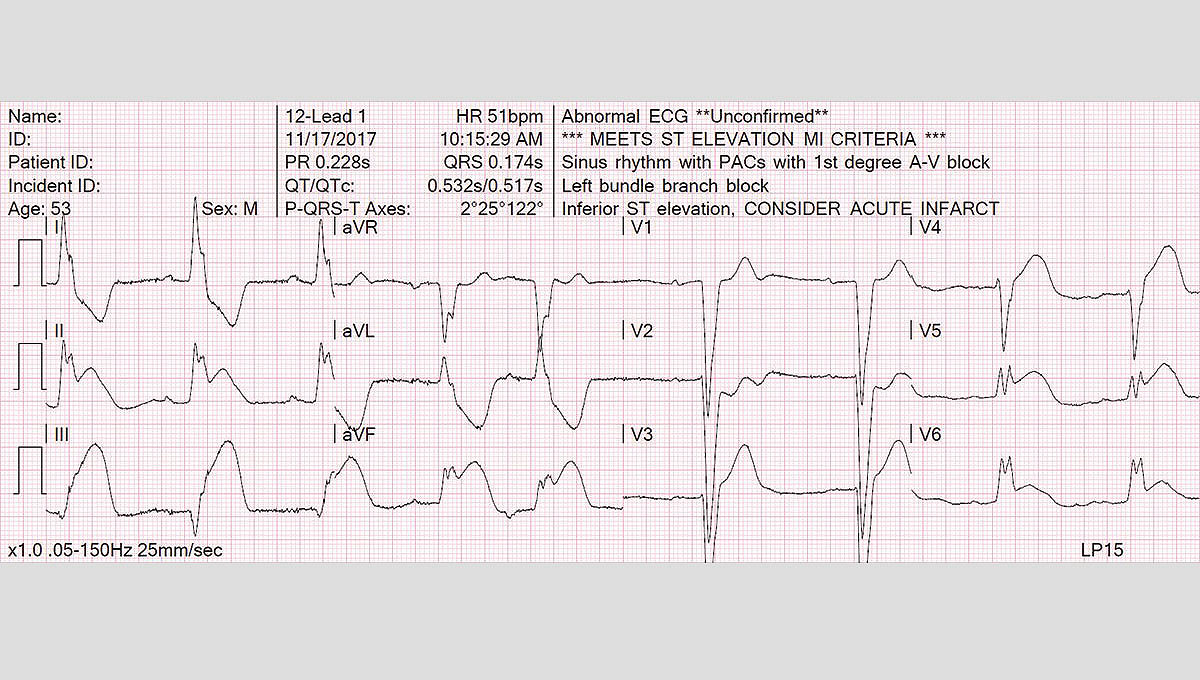
Uses a top 12-lead ECG algorithm
- Trusted, proven University of Glasgow 12-Lead ECG Analysis Program
- Continuous monitoring of all 12-leads with alerts when significant ST segment changes are detected
- Uses Sgarbossa criteria and measures STJ at the J point for possible STEMI patients and provides peadiatric interpretation for peadiatric patients.

LIFEPAK TOUGH
- Corner guards, shock-absorbing handle, and reinforced cable connections
- Dual-layer, anti-scratch screen
- Withstands severe impacts and extreme vibration
Jeffrey Goodloe, MD, NREMT-P, FACEP
Medical Director for Medical Control Board Oklahoma City-Tulsa area EMS system
Take the next step
Want to talk to your Sales Representative? Please complete the form below and we’ll connect you with your local representative.
When you invest in our lifesaving devices and data products, you are buying more than quality design and manufacturing—you’re also buying our unrivaled commitment to customer service and support.
Technical support
We offer one of the largest networks of technical service representatives in the industry. We’ll work with you to quickly assess any issues and find the best solution.
Training and education
As your lifesaving partner, Stryker offers training support, seminars, podcasts, and access to clinical research on the latest resuscitation strategies and tips.
Related products
LUCAS 3, v3.1 chest compression system
Deliver guidelines-consistent, high-quality chest compressions with less strain, micromanagement, and risk for the caregiver. The LUCAS chest compression system provides benefits both to the cardiac arrest patient and the resuscitation team.
Learn moreLIFENET System
The LIFENET System is a comprehensive cloud-based platform that seamlessly manages and delivers patient information and device data that EMS and hospital teams need to work together effectively.
Learn moreSources
1. Stiell I, Walker R, Nesbitt L, et al. Biphasic trial a randomized comparison of fixed lower versus escalating higher energy levels for defibrillation in out-of-hospital cardiac arrest. Circulation. 2007;115:1511-1517.
2. Walker G, Koster R, Sun C, et al. Defibrillation probability and impedance change between shocks during resuscitation from out-of-hospital cardiac arrest. Resuscitation. 2009;80:773-777.
3. Koster R, Walker R, Chapman F. Recurrent ventricular fibrillation during advanced life support care of patients with prehospital cardiac arrest. Resuscitation. 2008;78:252-257.
4. Khaykin Y, Newman D, Kowalewski M, et al. Biphasic versus monophasic cardioversion in shock-resistant atrial fibrillation: a randomized clinical trial. Journal of Cardiovascular Electrophysiology. 2003;8(14);868-872.
This document is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it.
The information presented is intended to demonstrate Stryker’s product offerings. A healthcare professional must always refer to operating instructions for complete directions for use indications, contraindications, warnings, cautions, and potential adverse events, before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your representative if you have questions about the availability of Stryker’s products in your area. Specifications subject to change without notice. The products depicted are CE marked in accordance with applicable EU Regulations and Directives.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: LIFEPAK, LIFENET, LUCAS, HealthEMS, RevNet, CODE-STAT, Physio-Control, HeartSine, samaritan, LIFEPAK CR, TrueCPR, REDI-CHARGE, QUIK-COMBO, EDGE, cprINSIGHT, ClearVoice, QUIK-STEP, LIFEPAK TOUGH, HomeSolutions.net, RELI, REDI-PAK, Shock Advisory System, LIFELINKcentral. All other trademarks are trademarks of their respective owners or holder. The yellow and black color scheme is a registered trademark of Stryker Corporation.
LIFEPAK devices: CE Class IIb (0123)
HeartSine device: CE Class IIb (0123)
LUCAS device: CE Class IIb (2460)
EC REP: Stryker European Operations Limited | Anngrove, IDA Business & Technology Park | Carrigtwohill, Co. Cork, T45 HX08 | Ireland
M0000007484 REV AA