LIFENET System
LIFENET System
Smooth communication. Fast care. Strong teamwork.
The LIFENET System is a comprehensive cloud-based platform that seamlessly manages and delivers patient information and device data that EMS and hospital teams need to work together effectively.

Take action with your data.
The LIFENET System manages and delivers patient and device data when it’s needed. Hospitals get advance notice of incoming critical patients. Care team members are activated in seconds to ensure patients receive timely care. Device readiness is tracked and managed effortlessly.

Minimize time to treatment for STEMI, stroke, and other emergencies
Faster response times lead to better outcomes. Give hospital critical care teams a head start on reducing time to treatment for all emergencies through comprehensive patient data delivery from EMS. Hospitals know exactly what to expect before patients reach their doors, which means care teams can be assembled faster and briefed in advance.

Enable quicker decisions and team collaboration
No matter where a physician might be located, their advice is only a click away with LIFENET Consult. This smartphone application allows clinicians to quickly perform remote consultations and decision support, while securely messaging the team coordinator.

Quickly mobilize data for reporting and quality improvement
In addition to capturing minute-by-minute transmission/reception history, the LIFENET System facilitates the immediate transfer of data to CODE-STAT data review software and ePCR solutions for efficient post-event QA/QI.
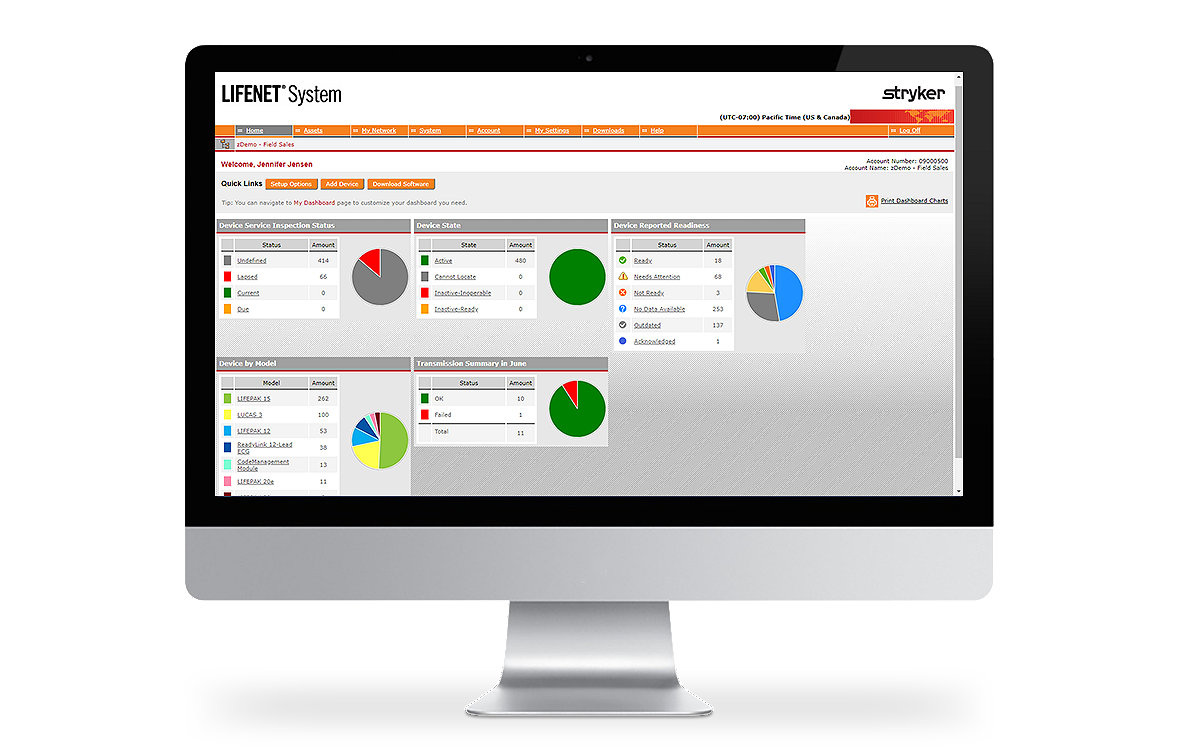
Easily manage equipment
The LIFENET System Asset allows you to manage an entire fleet of devices from one place. This web-based tool allows you to track equipment readiness, manage setup options, update software and receive notifications about potential device issues. Asset is highly configurable with automated alerting capabilities.

Protect patient privacy
The LIFENET System offers multiple security shields and audits to help hospitals and EMS teams protect patient health information.
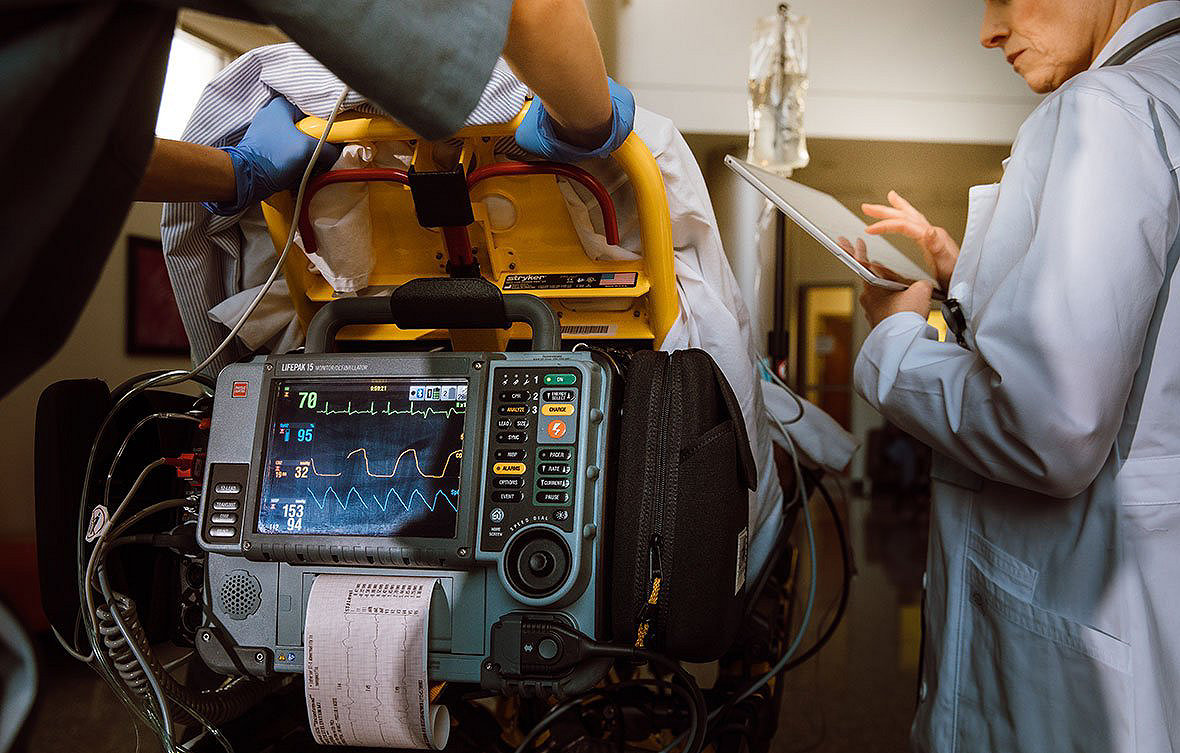
Trust a well established system
LIFENET is backed by a global player in emergency medicine and resuscitation, providing a comprehensive toolset for team coordination, alerting, data transmission, and device fleet management for multiple devices.
Product features
Alert
Never miss a notification again. Receive highly visible pre-arrival notifications and urgent patient information to facilitate transfer to definitive care.
Consult
Allow remote consultations and decision support through mobile devices, accelerating response to emergent patient data and increasing clinical efficiency.
OnePush
Automatically activate protocols for many emergent care needs, alert care teams to incoming patients, and timestamp specified events. All of this can be initiated by prehospital EMS transmission.
Connect
Send patient care data directly to CODE-STAT data review software or your ePCR directly from the LIFEPAK device through the same gateway used to transmit 12-lead ECGs.
Export
Provide a simple way to send 12-lead ECG data to a hospital’s electronic record system, such as GE MUSE®, for integration into the patient record.
Asset
Manage equipment status and service needs while coordinating configurations across all your equipment.
Take the next step
Want to talk to your Sales Representative? Please complete the form below and we’ll connect you with your local representative.
When you invest in our lifesaving devices and data products, you are buying more than quality design and manufacturing—you’re also buying our unrivaled commitment to customer service and support.
Technical support
We offer one of the largest networks of technical service representatives in the industry. We’ll work with you to quickly assess any issues and find the best solution.
Training and education
As your lifesaving partner, Stryker offers training support, seminars, podcasts, and access to clinical research on the latest resuscitation strategies and tips.
This document is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it.
The information presented is intended to demonstrate Stryker’s product offerings. A healthcare professional must always refer to operating instructions for complete directions for use indications, contraindications, warnings, cautions, and potential adverse events, before using any of Stryker’s products. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your representative if you have questions about the availability of Stryker’s products in your area. Specifications subject to change without notice. The products depicted are CE marked in accordance with applicable EU Regulations and Directives.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: LIFEPAK, LIFENET, LUCAS, HealthEMS, RevNet, CODE-STAT, Physio-Control, HeartSine, samaritan, LIFEPAK CR, TrueCPR, REDI-CHARGE, QUIK-COMBO, EDGE, cprINSIGHT, ClearVoice, QUIK-STEP, LIFEPAK TOUGH, HomeSolutions.net, RELI, REDI-PAK, Shock Advisory System, LIFELINKcentral. All other trademarks are trademarks of their respective owners or holder. The yellow and black color scheme is a registered trademark of Stryker Corporation.
LIFEPAK devices: CE Class IIb (0123)
HeartSine device: CE Class IIb (0123)
LUCAS device: CE Class IIb (2460)
EC REP: Stryker European Operations Limited | Anngrove, IDA Business & Technology Park | Carrigtwohill, Co. Cork, T45 HX08 | Ireland
M0000007484 REV AA




