false
/content/experience-fragments/stryker/corporate/en/about/news/homepage/news-homepage-hero/mobile


Latest news

Stryker launches T2 Alpha Humerus Nailing System, supporting surgical workflow and care for complex fractures
The system offers surgeons a streamlined approach to humeral fracture fixation while providing hospital teams with a unified platform that enables consistent, high-quality care.
Read More
Stryker introduces Mako Handheld Robotics with the limited market release of Mako RPS
Stryker announced the limited market release of Mako RPS (Robotic Power System) for Total Knee, an intuitive handheld robotic system that combines Stryker’s proven robotics and power tool legacies and represents Mako’s expansion into a new robotics platform.
Read More
Stryker names Spencer Stiles President and Chief Operating Officer
Stryker announced Spencer Stiles has been appointed President and Chief Operating Officer (COO), effective January 1, 2026. In this role, Stiles will lead the company’s global businesses, strategy, and mergers and acquisitions.
Read More
Stryker launches Incompass Total Ankle System at AOFAS 2025
Press release announcing Stryker will launch its Incompass™ Total Ankle System at the 2025 American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting in Savannah, Georgia, September 10–13, 2025
Read MoreFeatures

Three things to know about Stryker’s employee giving program
Learn more about Stryker’s employee giving program.
Read More
Hold on to the moments that matter
Sudden cardiac arrest can devastate our most precious moments. Watch our latest video and see how AEDs and fast action can help keep the picture whole and the future bright.
Read More
Survivor: John Jimenez
John Jimenez was at Seattle-Tacoma International Airport when, without warning, he collapsed after getting off the tram. Thanks to the airport’s accessible AEDs and the quick thinking of those around him, John’s life was saved.
Read More?$preset_307_184$)
Stryker focuses on and celebrates sustainability
Learn about Stryker’s Sustainability Solutions accomplishments as a “single-use” medical device reprocessor.
Read MoreFilter by:

11-Feb-2026
Stryker launches T2 Alpha Humerus Nailing System, supporting surgical workflow and care for complex fractures
The system offers surgeons a streamlined approach to humeral fracture fixation while providing hospital teams with a unified platform that enables consistent, high-quality care.
Read More
09-Feb-2026
Stryker introduces Mako Handheld Robotics with the limited market release of Mako RPS
Stryker announced the limited market release of Mako RPS (Robotic Power System) for Total Knee, an intuitive handheld robotic system that combines Stryker’s proven robotics and power tool legacies and represents Mako’s expansion into a new robotics platform.
Read More
04-Dec-2025
Stryker names Spencer Stiles President and Chief Operating Officer
Stryker announced Spencer Stiles has been appointed President and Chief Operating Officer (COO), effective January 1, 2026. In this role, Stiles will lead the company’s global businesses, strategy, and mergers and acquisitions.
Read More
25-Nov-2025
Three things to know about Stryker’s employee giving program
Learn more about Stryker’s employee giving program.
Read More
18-Nov-2025
What does an operating room look like? Look inside Stryker’s iSuite
Learn how Stryker’s iSuite custom operating rooms are designed to help make your surgical experience safer and more efficient
Read More
24-Oct-2025
Hold on to the moments that matter
Sudden cardiac arrest can devastate our most precious moments. Watch our latest video and see how AEDs and fast action can help keep the picture whole and the future bright.
Read More
26-Sep-2025
Survivor: John Jimenez
John Jimenez was at Seattle-Tacoma International Airport when, without warning, he collapsed after getting off the tram. Thanks to the airport’s accessible AEDs and the quick thinking of those around him, John’s life was saved.
Read More
09-Sep-2025
Stryker launches Incompass Total Ankle System at AOFAS 2025
Press release announcing Stryker will launch its Incompass™ Total Ankle System at the 2025 American Orthopaedic Foot & Ankle Society (AOFAS) Annual Meeting in Savannah, Georgia, September 10–13, 2025
Read More
29-Jul-2025
Inari Medical, now part of Stryker, launches the all-new InThrill® Thrombectomy System, designed to address arteriovenous access and small vessel thrombus cases
This is the first and only purpose-built small vessel and arteriovenous (AV) access thrombectomy system that can deliver fast, full luminal clot removal.
Read More
22-Jul-2025
Stryker launches Surpass Elite® Flow Diverter in the U.S., South Korea and Europe
This next-generation device represents a significant advancement in flow diversion, combining an upgraded implant design with a proprietary surface modification for enhanced performance and reduced thrombin generation.
Read More
25-Jun-2025
Stryker receives FDA clearance for Incompass Total Ankle System
Stryker's Incompass™ Total Ankle System is an implant intended for patients with ankle joints damaged by severe rheumatoid, post-traumatic, or degenerative arthritis.
Read More
23-Jun-2025
Stryker unveils new intracranial base catheter to streamline neurovascular access and therapy delivery
Stryker announced the launch of its AXS Lift Intracranial Base Catheter, a novel device developed to simplify neurovascular access and enhance delivery of interventional therapies.
Read More
23-Jun-2025
Innovation in hospital fall prevention
Stryker’s fall prevention solutions are designed to take some of the pressure off healthcare teams by using our smart, connected innovations.
Read More?$preset_666_392$)
05-Jun-2025
Stryker focuses on and celebrates sustainability
Learn about Stryker’s Sustainability Solutions accomplishments as a “single-use” medical device reprocessor.
Read More
23-May-2025
Meet Merryl, a resilient stroke survivor
Merryl dismissed early warning signs of stroke for nearly two days. As a survivor, he is passionate about raising awareness of the critical importance of seeking immediate medical attention after experiencing any stroke symptoms.
Read More
19-May-2025
Stryker receives FDA clearance for OptaBlate® BVN Basivertebral Nerve Ablation System
Stryker’s first basivertebral nerve ablation system targets the basivertebral nerve to provide relief from chronic* vertebrogenic low back pain.
Read More
13-May-2025
Advancing neurosurgery: Stryker supports brain tumor research through ongoing partnership
Learn how Stryker's Neurosurgical team is partnering again with the National Brain Tumor Society to help raise awareness and funds for brain tumor research.
Read More
04-Apr-2025
Stryker gets medical equipment to regions in need with Project C.U.R.E. partnership
Stryker partners with Project C.U.R.E. to get medical equipment and supplies to under-resourced nations.
Read More
01-Apr-2025
Stryker completes sale of U.S. spinal implants business
Stryker (NYSE: SYK), a global leader in medical technologies, announced today that it has completed the sale of its U.S. spinal implants business to Viscogliosi Brothers, LLC, as part of the newly formed company VB Spine, LLC.
Read More
01-Apr-2025
Stryker releases 2024 Comprehensive Report, highlighting a year of innovation, impact and growth
Stryker is pleased to announce the publication of our 2024 Comprehensive Report, showcasing another year of strong financial performance, innovation, and progress toward our corporate and sustainability commitments.
Read More
11-Mar-2025
Stryker showcases next generation of Mako SmartRobotics™ at AAOS 2025 Annual Meeting
Stryker showcases the latest advancements in Mako SmartRobotics™ across hip, knee, spine and shoulder procedures at the American Academy of Orthopaedic Surgeons’ (AAOS) 2025 Annual Meeting in San Diego.
Read More
06-Mar-2025
Inari Medical, now part of Stryker, launches Artix™ Thrombectomy System
Purpose-built for the distinct needs of the peripheral arterial system, Artix is a combined aspiration plus mechanical thrombectomy solution that delivers procedural control and versatility, and is designed to set a new standard for arterial thrombectomy.
Read More
04-Mar-2025
Stryker launches Steri-Shield 8 personal protection system
Stryker announced the launch of its Steri-Shield 8 personal protection system, the latest in its personal protective equipment (PPE).
Read More
03-Mar-2025
Stryker launches Sync Badge
Hands-free, wearable communication device supports care team members with fast and reliable collaboration.
Read More
19-Feb-2025
Stryker completes acquisition of Inari Medical, Inc., providing entry into the high-growth peripheral vascular segment
Stryker announced today that it has completed the acquisition of Inari Medical, Inc., a company that provides innovative solutions for venous thromboembolism (VTE) clot removal without the use of thrombolytic drugs.
Read More
12-Feb-2025
Enhancing hospital communication for patient and family satisfaction
The hospital experience can be stressful for patients and families. When a patient is undergoing a procedure, timely communication with patients and families helps relieve the anxiety of waiting and wondering.
Read More
31-Jan-2025
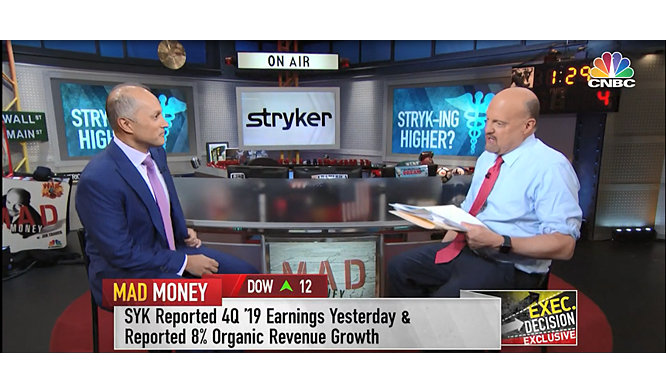
Watch Chair and CEO Kevin Lobo on CNBC’s Mad Money
Stryker Chair and CEO, Kevin Lobo, joins CNBC’s Mad Money with Jim Cramer to discuss our 2024 performance, excitement around our Mako Shoulder application and the overall strength of our diverse portfolio driving our company’s continued growth.
Read More
06-Jan-2025
Stryker announces definitive agreement to acquire Inari Medical, Inc., providing entry into high-growth peripheral vascular segment
Stryker announced today a definitive agreement to acquire all of the issued and outstanding shares of common stock of Inari Medical, Inc. (NASDAQ: NARI) for $80 per share in cash, representing a total fully diluted equity value of approximately $4.9 billion.
Read More
17-Dec-2024
Stryker honored with HIRC’s gold badge for resiliency
Stryker has been awarded the gold-level Healthcare Industry Resilience Collaborative (HIRC) resiliency badge, which recognizes organizations that demonstrate exceptional adaptability and performance within the healthcare supply chain.
Read More
03-Dec-2024
Stryker's Global Volunteer Network highlights importance of volunteerism
Learn how Stryker’s Global Volunteer Network connects passionate employees with volunteer opportunities.
Read More
19-Nov-2024
Stryker unveils Oculan Lighting Platform to enhance surgical visualization and illumination
Stryker unveiled the Oculan Lighting Platform, an innovative lighting solution designed to provide consistent, high-quality illumination, allowing surgeons to focus on delivering the highest standard of care.
Read More
14-Nov-2024
Stryker launches next generation of SurgiCount+ to help improve the standard of care in hospitals for sponge management and blood loss assessment
Stryker announced its launch of the next generation of SurgiCount+ within its sponge management portfolio.
Read More
24-Oct-2024
What are the symptoms and signs of stroke?
Learn how to spot the signs of stroke using the B.E. F.A.S.T. acronym.
Read More
23-Oct-2024
Five things you need to know about high blood pressure and stroke
Learn about the connection between high blood pressure and stroke, and our focus on advancing the practice of less invasive stroke therapies.
Read More
22-Oct-2024
Stryker and Operation Smile transform confidence and care
Learn how, for over 30 years, Stryker has partnered with Operation Smile to make healthcare better.
Read More
22-Oct-2024
How Stryker improves access to healthcare globally
Learn how Stryker is committed to improving patient outcomes by increasing access to safe and affordable healthcare globally.
Read More
01-Oct-2024
Stryker completes acquisition of Vertos Medical, Inc., expanding interventional pain management solutions
Stryker announced today that it has completed the acquisition of Vertos Medical Inc., a leader in interventional pain management solutions for chronic lower back pain caused by lumbar spinal stenosis.
Read More
20-Sep-2024
Stryker completes acquisition of NICO Corporation, expanding minimally invasive solutions for brain tumor removal and stroke care
Stryker announced that it has completed the acquisition of NICO Corporation, a privately held company providing a systematic approach to minimally invasive surgery for tumor and intracerebral hemorrhage (ICH) procedures.
Read More
17-Sep-2024
Stryker completes acquisition of care.ai
Stryker announced that it has completed the previously announced acquisition of care.ai. The acquisition will strengthen Stryker’s growing healthcare IT offering and wirelessly connected medical device portfolio.
Read More
11-Sep-2024
Stryker expands Foot & Ankle portfolio with specialized offerings designed to enhance surgical precision and outcomes
Stryker announced the addition of two new products to its Foot & Ankle portfolio – the Osteotomy Truss System™ (OTS) and Ankle Truss System™ (ATS), recently acquired from 4WEB Medical.
Read More?$preset_666_392$)
10-Sep-2024
How Stryker is innovating in operating room design
Discover how partnering with Stryker now gives you access to the Fortress modular OR wall system.
Read More
22-Aug-2024
Stryker announces definitive agreement to acquire Vertos Medical, Inc.
Stryker announced a definitive agreement to acquire Vertos Medical Inc., a privately held company providing a minimally invasive solution for treating chronic lower back pain caused by lumbar spinal stenosis.
Read More
21-Aug-2024
Employees share why DE&I matters at annual Summit
Learn how Stryker employees are using what they learned at the 2024 Summit to advance DE&I.
Read More
20-Aug-2024
A partnership promoting mental health for healthcare workers
Stryker partners with the Dr. Lorna Breen Heroes’ Foundation to support healthcare workers' mental health and wellbeing, improve access to mental health resources & prevent suicide.
Read More
12-Aug-2024
Stryker announces definitive agreement to acquire care.ai, a leading virtual care and ambient intelligence solutions platform
Stryker (NYSE: SYK), a global leader in medical technologies, announced today a definitive agreement to acquire care.ai, a privately held company specializing in delivering AI-assisted virtual care workflows, smart room technology and ambient intelligence solutions.
Read More
06-Aug-2024
Stryker announces launch of Pangea Plating System
New system enhances trauma care for surgeons with reliable and user-friendly solutions.
Read More
02-Aug-2024
Watch Chair and CEO Kevin Lobo on CNBC’s Money Movers
Stryker Chair and CEO, Kevin Lobo, joins CNBC’s Money Movers with Carl Quintanilla and Sara Eisen to discuss our company’s historic growth.
Read More
31-Jul-2024
Stryker completes acquisition of MOLLI Surgical
Stryker (NYSE:SYK), a global leader in medical technologies, announced today that it has completed the acquisition of MOLLI Surgical Inc., a privately held company specializing in the development of wire-free soft tissue localization technology for breast conserving surgery.
Read More
30-Jul-2024
Stryker’s Spine Guidance 5 Software featuring Copilot receives 510(k) clearance from FDA
Q Guidance System with Spine Guidance 5 Software featuring Copilot is a first-to-market technology, pioneering new planning and surgical capabilities
Read More
25-Jul-2024
Back pain and scoliosis: Victoria finds relief
Learn how Dr. George corrected Victoria’s scoliosis using the Everest Spinal System.
Read More
17-Jul-2024
Stryker’s Pierce shares how our latest digital innovations help caregivers and their patients
Andy Pierce, our Group President of MedSurg and Neurotechnology, joins the StrykerTalks podcast to share updates on how our advanced devices and software – such as Vocera technology and the 1788 camera – help our customers address some of their most pressing challenges across the continuum of care.
Read More
16-Jul-2024
For third consecutive year, Stryker named 2024 Best Place to Work for Disability Inclusion
News announcing Stryker has been once again named a top-scoring business on the Disability Equality Index® (DEI) by the nation’s largest disability rights organization, the American Association of People with Disabilities, and Disability:IN, the global business disability inclusion network, for our efforts to advance inclusion for people with disabilities.
Read More

18-Jun-2024
Stryker joins Atrium Health affiliate IRCAD North America to advance surgical training and education
Read More
13-Jun-2024
How Stryker supports the Red Cross' lifesaving mission
Learn how Stryker is helping the American Red Cross to prevent and alleviate human suffering.
Read More
10-Jun-2024
Stryker boosts innovation: R&D expands with medical device testing facility
Stryker's Global Technology Centre (SGTC) has recently expanded to accommodate rigorous life cycle testing of medical devices, adding highly skilled engineers and microbiologists to conduct expanded testing.
Read More
04-Jun-2024
Stryker releases LIFEPAK 35 monitor/defibrillator
LIFEPAK 35 monitor/defibrillator offers advanced technology to help move patient care forward.
Read More
03-Jun-2024
Stryker announces definitive agreement to acquire Artelon, Inc., offering a range of soft tissue fixation solutions for orthopaedic surgeons
Read More
06-May-2024
Nurses working at Stryker share how their experience becomes innovation in nursing
Learn how nurses at Stryker are at the forefront of innovation to improve outcomes for patients.
Read More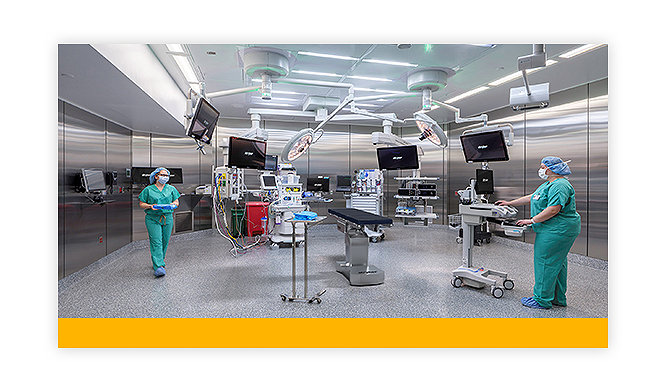

24-Apr-2024
Listen to Stryker Chair and CEO Kevin Lobo on Ellig Group’s Leadership Reimagined Podcast
Read More
24-Apr-2024
Stryker receives Don Clifton Strengths-Based Culture Award for fifth year in a row
Read More


01-Apr-2024
Stryker releases Comprehensive Report highlighting 2023 performance and progress towards environmental, social and governance (ESG) commitments
Stryker is pleased to announce the publication of our 2023 Comprehensive Report which covers our 2023 financial performance and progress on our environmental, social and governance goals.
Read More
21-Mar-2024
Three ways Stryker supports physicians in their pursuit of better patient outcomes
With more than 900,000 active physicians in the United States, training and education opportunities are integral in a physician’s ability to improve patient outcomes.
Read More


12-Feb-2024
How augmented reality can help care teams plan, design and construct new operating rooms
Augmented reality allows Stryker’s Communications team to work with customers to help them envision their ideal operating rooms.
Read More

31-Jan-2024
Stryker expands Prophecy® Surgical Planning system to include the new Footprint™, offering surgeons a comprehensive view of the foot and ankle
Read More
17-Jan-2024
New intermediate nail and RC Lag Screw expand Gamma4 portfolio to meet the needs of surgeons and their patients
Read More
16-Jan-2024
How Janet recovered from a vertebral (spine) compression fracture
Janet worked with Dr. Smith to learn about the treatment options for her vertebral compression fracture, or ‘VCF’ for short.
Read More
11-Jan-2024
Katherine’s catastrophic stroke survivor story
Meet Katherine Wolf, a stroke survivor who suffered from a catastrophic stroke when she was just 26 years old.
Read More
10-Jan-2024
First Shoulder Arthroplasty Surgeries Using Stryker’s Blueprint® Mixed Reality Guidance System Completed at Mayo Clinic in Minnesota and at St. Joseph’s Health Care London in Canada
Read More
18-Dec-2023
Stryker announces intent to acquire SERF SAS, enhancing its global joint replacement leadership
Read More


31-Oct-2023
Stryker’s Corporate Responsibility VP talks social, environmental strategy in MedTech on The Impact Podcast
Read More
27-Oct-2023
New SPY-PHI technology* to help breast cancer surgeons see more during surgery
Read More

12-Oct-2023
Over 2,400 surgical cases completed using Stryker’s Q Guidance System with Spine Guidance Software in first year
Read More

09-Oct-2023
Delivering a more efficient experience for surgeons and patients in ambulatory surgery centers
Read More


03-Oct-2023
Knowing the warning signs of sudden cardiac arrest helped saved his life | Derrick’s sudden cardiac arrest story
Read More

21-Sep-2023
Stryker’s Triathlon Tritanium Baseplate marks a decade of positive patient impact
Read More
19-Sep-2023
Stryker Launches PROstep® MIS Lapidus, a New Minimally Invasive Surgical Option for Treating Patients with Bunions
Read More

14-Sep-2023
A Clearer Vision of Surgery: Stryker Releases its Next-Generation of Advanced Surgical Cameras
Read More

31-Aug-2023
Endoscopy’s Rosales shares how we continue innovating in surgical visualization
Read More
28-Aug-2023
Stryker’s third-annual DE&I Summit: Ensuring every employee is seen, heard and valued
Read More

04-Aug-2023
Stryker launches national direct-to-patient marketing campaign
“Scan. Plan. Mako Can.” spotlights and differentiates Stryker’s Mako SmartRobotics™ for partial knee, total knee and total hip replacement surgery
Read More

11-Jul-2023
Stryker named 2023 Best Place to Work for Disability Inclusion for second consecutive year
Read More
11-Jul-2023
Stryker announces commercial launch of Q Guidance System with Cranial Guidance Software
Read More
06-Jul-2023
Mako: Robotics driving innovation across specialties
Stryker's Vice President & General Manager, Mako, gets detailed about Mako Systems
Read More


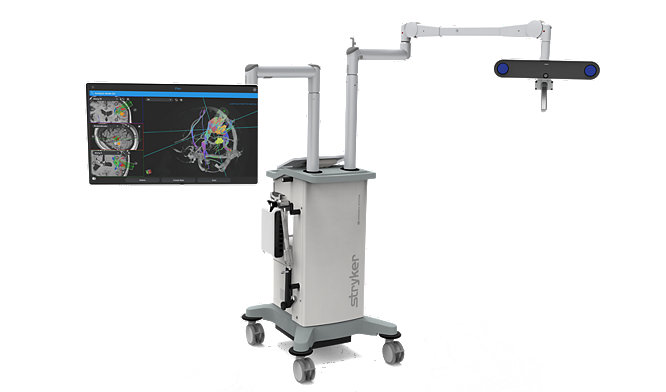
24-May-2023
Surgeons successfully complete first surgical cases using Stryker’s Q Guidance System with Cranial Guidance Software
Read More
?$preset_666_392$)
12-May-2023
Stryker awarded Transparency Partner distinction by the Healthcare Industry Resiliency Collaborative
Read More



?$preset_666_392$)

21-Apr-2023
Lin's battle with stage 3 colon cancer
Learn how a trusting relationship with a doctor, the right technology, and the support of his family helped guide Lin to remission.
Read More?$preset_666_392$)


12-Apr-2023
Stryker’s Lobo discusses outlook for our business, M&A strategy and future product innovation
Read More
31-Mar-2023
Stryker releases Comprehensive Report highlighting 2022 performance and progress against environmental, social and governance (ESG) commitments
Read More
30-Mar-2023
Stryker now offers the only FDA-cleared pyrocarbon bearing material option for shoulder hemiarthroplasty
Read More





06-Mar-2023
Stryker’s Heath shares three commitments to building diversity in workplace, supplier base and beyond
Read More
01-Mar-2023
CD NXT power tool system empowers surgeons through real-time depth measurement
Read More
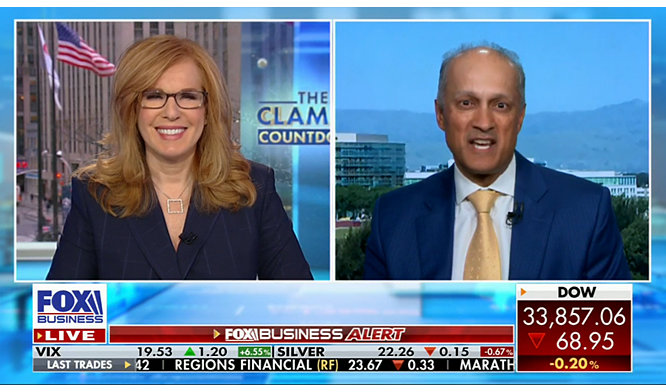


27-Jan-2023
Stryker’s Cohen delves into transformative nature of surgical robotics and digital tech
Read More
19-Jan-2023
Advancing healthcare professionals’ education with Stryker’s Neurotech Mobile Experience
Read More
20-Dec-2022
Stryker donates to K9s For Warriors to conclude 2022 Minor League Baseball activation
Read More
19-Dec-2022
Stryker’s Pierce highlights how we continue innovating toward safer care, better outcomes
Read More
15-Dec-2022
Stryker fulfills carbon reduction goal early, commits to 100% renewable energy by 2027
Read More
13-Dec-2022
Stryker launches Citrefix™ Suture Anchor System, featuring award-winning Citregen biomaterial designed to support bone regeneration and the natural healing process
Read More


15-Nov-2022
ENT’s Stewart shares views on leadership, Stryker’s Women’s Network and vital business opportunities
Read More
14-Nov-2022
Stryker opens the OR of the Future for customers to experience tomorrow’s OR today
Read More


12-Oct-2022
Stryker to Showcase Innovative Portfolio of Spine Solutions at the North American Spine Society Meeting
Read More




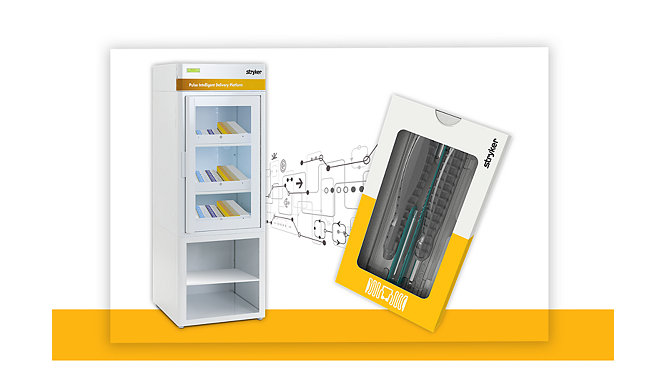
14-Sep-2022
Stryker Launches Pulse Intelligent Delivery Platform at the 2022 American Orthopaedic Foot & Ankle Society Annual Meeting
Read More
07-Sep-2022
ESG lead Buckley explains how Stryker operates sustainably and responsibly and embraces change
Read More



23-Aug-2022
How Stryker built its trauma and extremities business through innovation and acquisition
Read More

03-Aug-2022
Stryker advances its global additive manufacturing leadership with new facility at Anngrove, creating capacity for 600 additional jobs
Read More

01-Aug-2022
John Collings named chair of Asia Pacific Medical Technology Association (APACMed)
Read More
08-Jul-2022
Tour the Amsterdam Skills Centre, an international training centre for medical specialists
Read More
28-Jun-2022
Stryker Launches “Free Your Feet” Campaign to Raise Awareness About New Bunion Treatments
Read More


07-Jun-2022
Stryker strengthens its R&D capabilities with advanced Global Technology Center
Read More
06-Jun-2022
Why Mark Paul believes we’re entering into “Golden Era” of neurovascular tools and new technologies
Read More
02-Jun-2022
Is it a heart attack or sudden cardiac arrest? Know the warning signs and what to do for both
Read More

25-May-2022
Stryker Launches EasyFuse™ Dynamic Compression System for High-Demand Foot and Ankle Applications
Read More

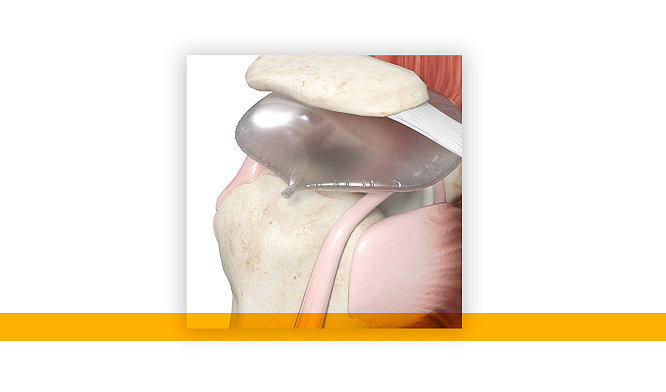
03-May-2022
Stryker’s InSpace subacromial balloon spacer two-year, Level 1 randomized controlled clinical study published in the Journal of Bone and Joint Surgery
Read More


12-Apr-2022
Acute Care's Mathieson explains how Vocera fits into Stryker's goal to help nurses
Read More
11-Apr-2022
CMS grants outpatient facility code for insertion of Stryker’s InSpace subacromial balloon spacer
Read More
01-Apr-2022
Stryker releases Comprehensive Report highlighting 2021 performance and progress against environmental, social and governance (ESG) commitments
Read More
30-Mar-2022
Three ways Stryker works to support physicians in their pursuit to improve patient outcomes
Read More

22-Mar-2022
Stryker Launches Insignia® Hip Stem at the American Academy of Orthopaedic Surgeons (AAOS) 2022 Annual Meeting
Read More

15-Mar-2022
Stryker works with Red Cross to direct resources to humanitarian efforts in Ukraine
Read More
14-Mar-2022
Stryker’s Stiles shares the art and science used in integrating Wright Medical as well as the future of Mako
Read More

01-Mar-2022
Vocera, now part of Stryker, introduces latest evolution in hands-free communication to help protect and connect healthcare workers
Read More
24-Feb-2022
Stryker Launches PROstep™ MICA® SOLO Guide to Simplify Minimally Invasive Bunion Procedures
Read More


16-Feb-2022
2022 ANA Innovation Award Winners Address Gaps in Health Equity and Education Brought on by COVID-19
Read More




20-Jan-2022
Stryker Announces First Patient Case Using New Tornier Perform® Patient-Matched Primary Reversed Glenoid
Read More





30-Nov-2021
Stryker honored by Women’s Forum for advancing gender representation in the boardroom
Read More




04-Nov-2021
Stryker expands its Mako SmartRobotics footprint to reach more veterans and military suffering from joint pain
Read More



01-Oct-2021
'I can’t change my [breast cancer] diagnosis, but I can change what I do about it'
Read More

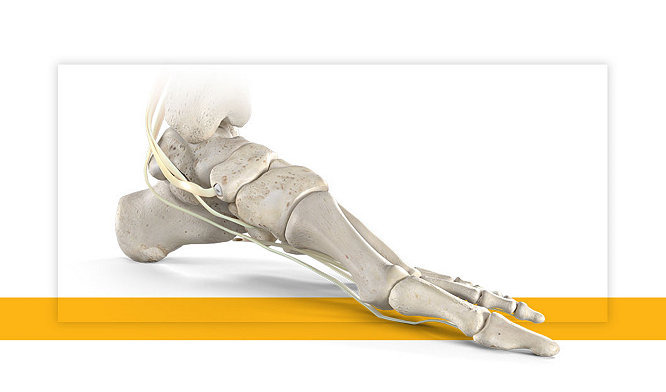








03-Aug-2021
Being in the right place at the right time | Matt’s sudden cardiac arrest story
Read More

30-Jul-2021
Stryker Sponsors the PGA TOUR's 2021 World Golf Championships-FedEx St. Jude Invitational and Educates Fans on Joint Health
Read More

14-Jul-2021
Stryker announces the FDA clearance of the first biodegradable subacromial balloon spacer, filling a gap in the shoulder continuum of care
Read More
13-Jul-2021
Stryker Introduces Market-Leading Tornier Shoulder Arthroplasty Portfolio and Launches New Tornier Perform™ Humeral System
Read More


18-May-2021
Stryker to Showcase New, Combined Foot and Ankle Portfolio at 2021 ACFAS Vegas Scientific Conference
Read More


29-Apr-2021
Stryker enters national partnership with Minor League Baseball to become the “Official SmartRobotics™ Joint Replacement Partner”
Read More?$preset_666_392$)

?$preset_666_392$)



27-Jan-2021
Employees inspire a new generation of robotics engineers through Discovery Channel’s BattleBots
Read More
11-Jan-2021
Kevin Lobo shares leadership strategies with Medical Design & Outsourcing, DeviceTalks
Read More

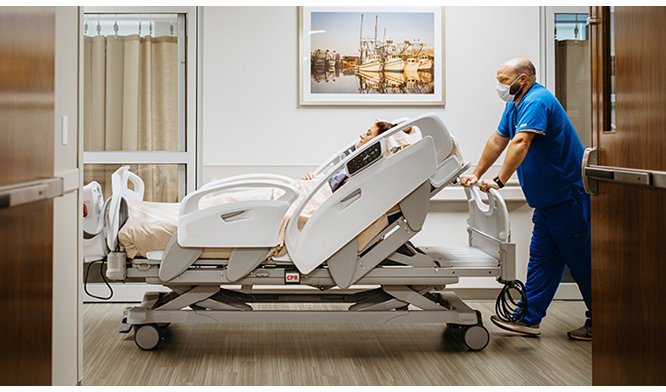
08-Dec-2020
Innovation inspired by insights: the next generation, first completely wireless hospital bed is here
Read More
04-Dec-2020
CMS grants transitional pass-through payment (TPT) for Stryker’s SpineJack® System
Read More


20-Oct-2020
Awareness campaign launched to support World Restart a Heart Day and World Stroke Day
Read More


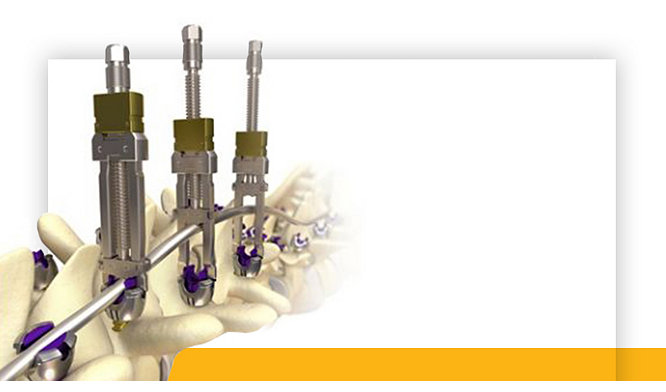
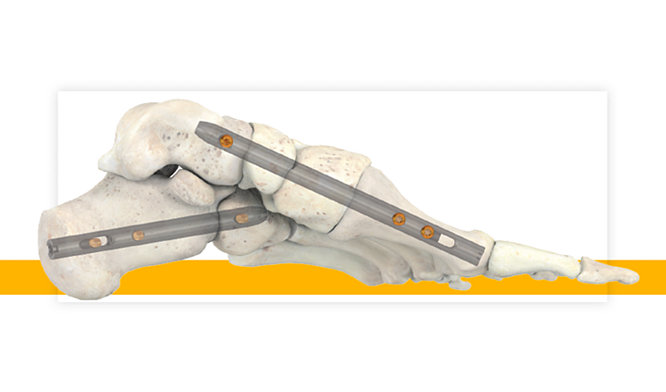
28-Sep-2020
Stryker’s T2 ICF Nail System Offers Surgeons New Option for Patients Suffering from Serious Foot Conditions
Read More
21-Sep-2020
CMS grants new technology add-on payment (NTAP) for Stryker’s SpineJack® System
Read More




01-Sep-2020
Stryker’s AxSOS 3® Ankle Fusion System offers innovative plates that are designed to fit a range of patients
Read More
24-Aug-2020
Stryker launches first 64-wire cobalt chromium flow diverter in the US to treat brain aneurysms
Read More

03-Aug-2020
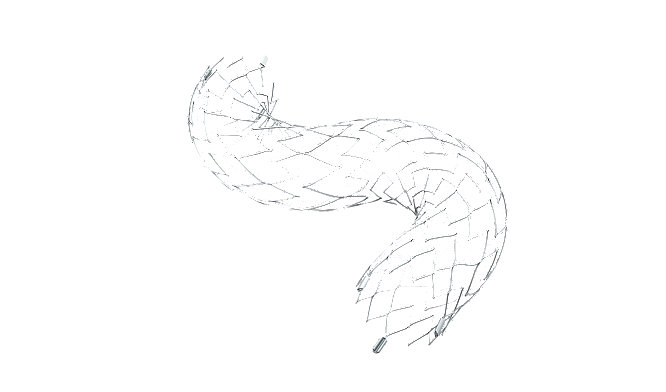
Stryker’s Neuroform Atlas® Stent System granted an expanded indication, providing a new option for patients with aneurysms in the back of the brain
Read More












03-Apr-2020
Stryker releases Emergency Relief Bed™, a limited-release medical bed to support critical needs during pandemic
Read More









01-Nov-2019
Stryker receives FDA clearance for SAHARA® Lateral 3D Expandable Interbody System
Read More

%20for%20customer%20lab%20in%20Kalamazoo?$preset_666_392$)
25-Sep-2019
Stryker announces SAKOS pivotal trial results for SpineJack System published in The Spine Journal
Read More
04-Sep-2019
Stryker announces definitive agreement to acquire Mobius Imaging & Cardan Robotics
Read More
04-Sep-2019
Stryker honored by Women’s Forum for work toward gender parity in the boardroom
Read More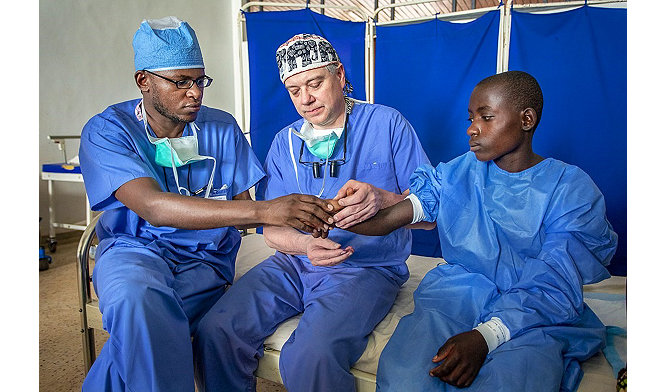


01-Jul-2019
Innovation and design in stroke technology. An interview with John Daniel, Vice President, Neurovascular R&D
Read More
01-Jul-2019
Trees, landfills and the environment: How we're partnering with customers to address sustainability
Read More24-Jun-2019
Stryker announces 33,000 patients have been treated with LATERA absorbable nasal implant
Read More02-May-2019
At AANA 2019, Stryker hosts a lunch-and-learn presentation featuring a new surgical planning and execution platform designed to enhance efficiency and patient experience for hip arthroscopy
Read More
15-Apr-2019
Stryker showcases new Sonopet iQ Ultrasonic Aspirator System at the American Association of Neurological Surgeons annual meeting
Read More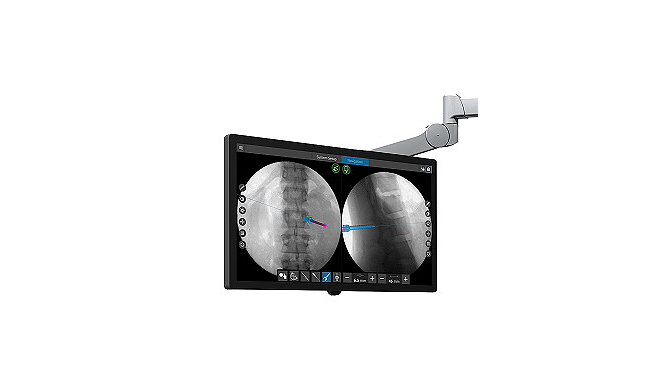
15-Apr-2019
Stryker features new SpineMap Go software at the American Association of Neurological Surgeons annual meeting this week
Read More09-Apr-2019
Stryker Launches LIFEPAK® CR2 Defibrillator with LIFELINKcentral™ AED Program Manager in the United States
Read More



12-Mar-2019
Stryker Announces Clinical Evidence Demonstrating Improved Early Functional Recovery with Mako Total Knee
Read More
11-Mar-2019
Stryker introduces new visualization platform designed to enhance orthopaedic surgical efficiency and the patient experience
Read More01-Feb-2019
Stryker Launches Voluntary Field Action for Specific Units of the LIFEPAK® 15 Monitor/Defibrillator
Read More?$preset_666_392$)
08-Nov-2018
Stryker's mobility zone makes final stop of 2018 at Charles Schwab Cup Championship
Read More22-Oct-2018
Thinking inside the box: Stryker sponsors entrepreneurial hackathon to solve a medical challenge
Read More08-Oct-2018
Stryker's Advanced Guidance Technologies announces partnerships with Synaptive Medical and Ziehm Imaging
Read More

26-Sep-2018
Stryker announces 510(k) clearance of SpineJack® Implantable Fracture Reduction System
Read More20-Sep-2018
Stryker’s Spine Division To Showcase Tritanium® In-Growth Technology in Augmented Reality Experience at NASS 2018
Read More04-Sep-2018
Stryker to participate in Morgan Stanley 16th Annual Global Healthcare Conference
Read More
23-Aug-2018
Stryker, The Official Joint Replacement Products Of The PGA TOUR®, Brings Joint Health Education To THE NORTHERN TRUST
Read More21-Aug-2018
Stryker Teams Up With Jerome Bettis and Fred Funk to Celebrate the Selection of the "Get on the Bus" Contest Winner in New Jersey
Read More02-Aug-2018
Newly Published Data Demonstrate Bone In-Growth Potential of Stryker’s 3D-Printed Tritanium® Cage
Read More
01-Aug-2018
Stryker earns two spots in “The Ten Best Sports Medicine Technologies for 2018” listing from Orthopedics This Week
Read More17-Jul-2018
Stryker and Fred Funk Host Second "Get on the Bus" Contest Winner in Jacksonville to Promote Importance of Joint Health
Read More16-Jul-2018
Stryker receives US FDA Pre-market approval for the Surpass Streamline™ Flow Diverter to treat large and giant unruptured aneurysms
Read More11-Jul-2018
Stryker Brings The Mobility Zone® To Constellation SENIOR PLAYERS Championship
Read More20-Jun-2018
Stryker Supports Veterans, Brings Mission Of Making Healthcare Better To The Travelers Championship
Read More12-Jun-2018
Stryker And Jerome Bettis Host First-Ever "Get On The Bus" Contest Winner In Pittsburgh To Promote Importance Of Joint Health
Read More17-May-2018
Stryker To Make Its Sixth K9s For Warriors Donation Of 2018 At The AT&T Byron Nelson
Read More02-May-2018
Stryker Brings The Mobility Zone® And Joint Health Education To The Wells Fargo Championship
Read More02-May-2018
AdvaMed Congratulates Stryker’s Bronwen Taylor on Receiving 2018 PwC MedTech Ethical Leadership Award
Read More?$preset_666_392$)
?$preset_666_392$)
18-Apr-2018
Stryker to showcase its groundbreaking Target Guided Surgery navigation system at the Combined Otolaryngology Spring Meetings
Read More10-Apr-2018
Stryker Launches Campaign Challenging Those Suffering From Joint Pain to "Get on the Bus" to a Healthier Lifestyle
Read More04-Apr-2018
AlloSource and Stryker Launch ProChondrix CR Cryopreserved Osteochondral Allograft
Read More
23-Mar-2018
Stryker F1™ Small Bone Power System brings fresh innovation to the power tool market
Read More15-Mar-2018
Stryker Spine Division’s Tritanium C Anterior Cervical Cage Gains Momentum With Surgeons
Read More14-Mar-2018
Stryker Brings The Mobility Zone™ And K9s For Warriors Ceremony To Arnold Palmer Invitational To Kick Off 2018 Tournament Schedule
Read More07-Mar-2018
Stryker’s Spine Division Receives FDA Clearance for 3D-Printed Tritanium TL Curved Posterior Lumbar Cage
Read More07-Mar-2018
Stryker unveils market's first reusable suture passer for arthroscopic rotator cuff repair, Cobra
Read More
06-Mar-2018
Stryker introduces groundbreaking, post-free distraction system, designed to mitigate common groin complications associated with hip arthroscopy
Read More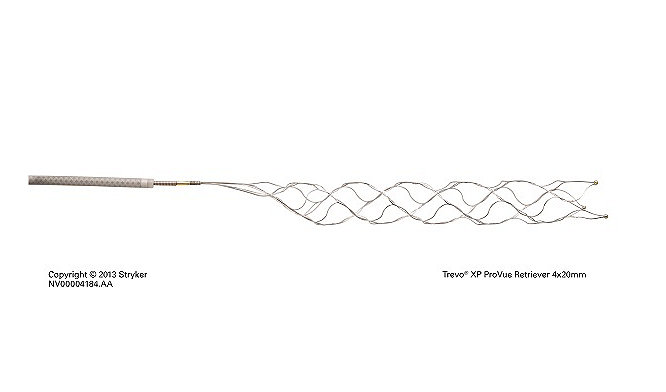
15-Feb-2018
Stryker’s Trevo® Retriever becomes first and only device indicated for treatment up to 24 hours, aligning with new AHA/ASA guidelines
Read More14-Feb-2018
Stryker crosses the 95% thresholds in VEXIM and announces its intention to file a proposed public buy-out offer followed by a squeeze-out for the outstanding shares of VEXIM
Read More26-Jan-2018
Stryker’s WEAVE Trial demonstrates positive results of endovascular treatment for patients with intracranial atherosclerotic disease
Read More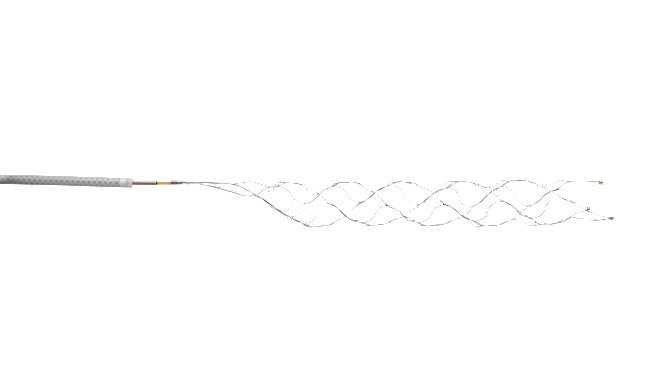
25-Jan-2018
Stryker’s DAWN Trial results contribute to updated acute ischemic stroke guidelines from the American Heart Association and American Stroke Association
Read More08-Jan-2018
Stryker announces nomination of two new Directors, Sheri S. McCoy and Rajeev Suri
Read More12-Dec-2017
Stryker named one of Great Place to Work’s “Best Companies to Work for in Greater China”
Read More13-Nov-2017
Stryker announces publication of the DAWN™ Trial results in the New England Journal of Medicine
Read More10-Nov-2017
Stryker earns a top score on the Human Rights Campaign Foundation’s Corporate Equality Index for LGBTQ Workplace Equality
Read More
09-Nov-2017
Stryker receives US FDA HDE approval for the Neuroform AtlasTM Stent System to treat wide neck aneurysms
Read More24-Oct-2017
Stryker’s Spine Division to Commercially Launch Serrato Pedicle Screw at NASS 2017
Read More
24-Oct-2017
Stryker enters agreement with Philips to help health systems drive down device costs, lower environmental footprint
Read More23-Oct-2017
Stryker’s Spine Division to Launch Tritanium® C Anterior Cervical Cage at NASS 2017
Read More
16-Oct-2017
Stryker Receives FDA Clearance for Cementless Mako Total Knee with Triathlon® Tritanium®
Read More
26-Sep-2017
Stryker’s Serrato Pedicle Screws Implanted by More Than 100 Surgeons in First Month, Prior to Full Commercialization
Read More
20-Sep-2017
Stryker’s Spine Division Receives FDA Clearance for 3D-Printed Tritanium® C Anterior Cervical Cage
Read More20-Sep-2017
Stryker Orthopaedics Debuts Final Episode of its Three-Part Series "Road Trip to a Healthier Lifestyle" featuring Jerome Bettis and Fred Funk
Read More14-Sep-2017
Get a sneak peek at how Stryker is expanding its advanced imaging capabilities with the addition of Novadaq
Read More23-Aug-2017
Stryker To Present Fifth K9s For Warriors Donation of 2017 at THE NORTHERN TRUST
Read More03-Aug-2017
Stryker Returns To 3M Championship For Its Third Year By Welcoming Fans To The Mobility Zone
Read More29-Jun-2017
Stryker Unveils Hoffmann LRF Hexapod With Pre And Post-Operative Planning Tool For Deformity Correction & Limb Reconstruction Procedures
Read More21-Jun-2017
Stryker To Make Its Fourth K9s For Warriors Donation Of 2017 at Travelers Championship
Read More06-Jun-2017
100,000th Robotic-Arm Assisted Joint Replacement Procedure Performed in the U.S. with Stryker's Mako System
Read More24-May-2017
Stryker returns to DEAN & DELUCA Invitational for second consecutive year with plans to honor military veteran with service canine
Read More18-May-2017
Stryker and AlloSource announce collaboration to distribute biologics for use in sports medicine procedures
Read More17-May-2017
Stryker debuts first episode of video series "Road Trip to a Healthier Lifestyle" featuring Jerome Bettis and Fred Funk
Read More16-May-2017
DAWN™ trial results demonstrate a 73% reduction in disability in stroke patients treated up to 24 hours
Read More21-Apr-2017
Stryker’s Spine division to feature comprehensive product portfolio at AANS 2017 Conference
Read More19-Apr-2017
Physio-Control launches HeartSine® samaritan® PAD 360P Automated External Defibrillator in United States
Read More30-Mar-2017
Stryker Orthopaedics announces "Road Trip to a Healthier Lifestyle" digital content series featuring Jerome Bettis and Fred Funk
Read More15-Mar-2017
Stryker’s System 8 Power Tools bring increased reliability, broadened offering to orthopaedic market
Read More14-Mar-2017
Stryker enabling surgeons to have a more predictable surgical experience with launch of Mako robotic-arm assisted total knee application
Read More08-Mar-2017
Stryker’s Spine division to feature novel 3D-printed spinal implants at AAOS Conference
Read More08-Mar-2017
Stryker announces an early stop to enrollment in the DAWN™ trial due to the high probability of trial success
Read More21-Feb-2017
Stryker chooses Microsoft HoloLens to bring operating room design into the future with 3D
Read More31-Jan-2017
Stryker’s Medical division announces launch of Altrix™ Precision Temperature Management System
Read More24-Jan-2017
Stryker brings Mobility Zone To Farmers Insurance Open to kick off 2017 Tournament Schedule, its first-ever activation in Southern California
Read More19-Dec-2016
Stryker Orthopaedics to compensate additional eligible U.S. patients who had surgery to replace their Rejuvenate Modular-Neck and/or ABG II Modular-Neck Hip Stems
Read More07-Dec-2016
Stryker increases 2017 annual dividend by 12% and declares a $0.425 per share quarterly dividend
Read More07-Dec-2016
Stryker increases 2017 annual dividend by 12% and declares a $0.425 per share quarterly dividend
Read More14-Nov-2016
Stryker announces multi-year partnership with Indo UK Institute of Health to deliver affordable orthopaedic care in India
Read More14-Nov-2016
Stryker announces multi-year partnership with Indo UK Institute of Health to deliver affordable orthopaedic care in India
Read More08-Nov-2016
Stryker Orthopaedics to honor veteran with donation of fourth service dog with K9s For Warriors at Charles Schwab Cup Championship
Read More28-Oct-2016
Study to be presented at NASS 2016 evaluating bone in-growth for Stryker’s 3D-Printed Tritanium® PL Cage
Read More26-Oct-2016
Stryker Mobility Zone heads to inaugural PowerShares QQQ Championship for Schwab Cup playoffs
Read More25-Oct-2016
Innovative product and service offering for the future of spinal surgery to be introduced NASS 2016
Read More06-Sep-2016
Stryker’s Trevo® Retriever is the first therapy in 20 years to be declared a front-line treatment to reduce disability in ischemic stroke
Read More31-Aug-2016
Stryker to participate in 11th Annual Wells Fargo Securities Healthcare Conference
Read More22-Aug-2016
Stryker Returns To The Barclays To Educate Golf Fans About Importance Of Joint Health And Staying Active
Read More12-Aug-2016
Stryker’s SurgiCount Safety Sponge System announces its risk sharing and indemnification program
Read More07-Jul-2016
Stryker Mobility Zone Makes Eighth Tour Stop of 2016 at DICK'S Sporting Goods Open
Read More28-Jun-2016
Jerome Bettis Tees Off Stryker Presence At 2016 World Golf Championships-Bridgestone Invitational
Read More22-Jun-2016
Stryker Mobility Zone Makes First-Ever Appearance At American Family Insurance Championship
Read More06-Jun-2016
Constellation SENIOR PLAYERS Next Stop For Stryker Orthopaedics On 2016 PGA TOUR Champions
Read MoreFor all investor relations news please visit the Investor Resources page.
SYK CORP 2022-03-34
