Training and Education You specialise in your patients, we specialise in you.

We are committed to partnering with you to deliver high quality personalised education programs, designed to advance surgical techniques.
Whether it’s at a local, national or European level, we are proud to provide a specialised educational experience.
As a healthcare professional, you can count on us to help contribute to your success through training focused on quality clinical and technical application.
A meaningful experience
________________
Make the most of your time. We strive to provide you with useful courses that optimise your learning.
An effective experience
________________
Stay in the know. Take what you learn here today, and apply it tomorrow.
A personalised experience
________________
We'll keep up. As you progress, so will our offerings.
Each year, our medical education team trains thousands of healthcare professionals.
Invested in you in
2023
Events
200+
HCPs trained
2600+

Stryker is a member of MedTech Europe, the European Industry association representing the medical technology industries, from diagnosis to cure.
As a member of MedTech Europe, we are committed to comply with the MedTech Europe Code of Ethical business Practices while partnering on educational opportunities.
For further information, please visit the MedTech Europe website.
We offer a full range of learning opportunities and are constantly developing new courses and training resources. From hands-on surgical trainings to virtual courses, we offer activities that address evolving clinical and operational challenges.
Surgical training lab
Cadaveric labs focused on procedure and product education.
Clinical workshops
Simulations and training with models or animal bones.
Speaker series
Didactic based education.
Virtual education
Live streaming educational events including surgery and symposiums.
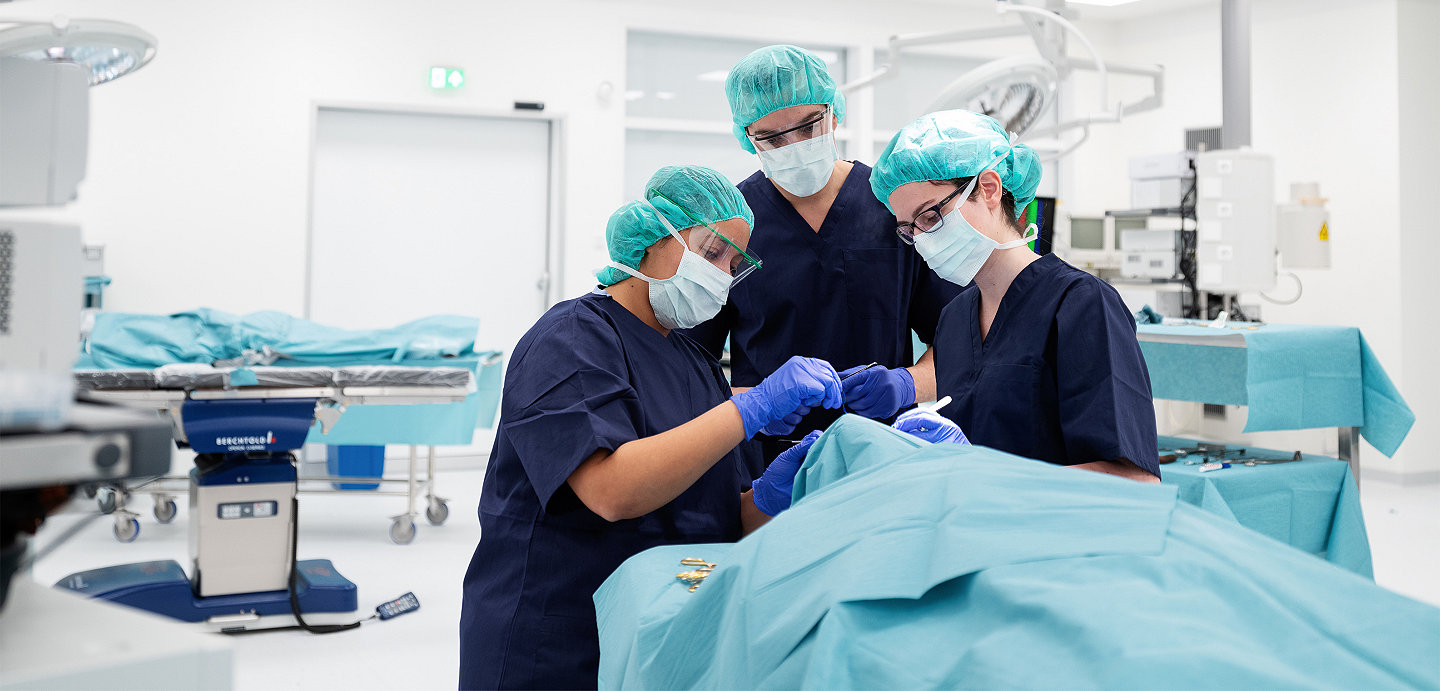
Our extensive Stryker course offering ranges from fundamental resident training to advanced surgical techniques, to meet your need for tiered levels of training and education.
Essential
_____________________________
Education and training for healthcare professionals in the first years of their specialisation.
The opportunity to participate in local courses and events that showcase the safe and effective use of Stryker products.
Advanced
_____________________________
For healthcare professionals in their final years as a trainee and first five years in pratice.
Advanced level educational opportunities that reflect their increased knowledge and experience.
Expert
_____________________________
For senior level healthcare professionals.
The ability to enhance their knowledge with a view to positively impact the future of surgery.
We are organising and supporting two types of educational events.
Stryker Educational Events: Educational events which are organised and run by Stryker, where we are responsible for the faculty, agenda and delegates.
Third Party Organised Educational Events: Educational events where a third party (usually the course director or a university) is responsible for the faculty, agenda and delegates. Stryker can provide support in the form of medical education equipment and grants.*
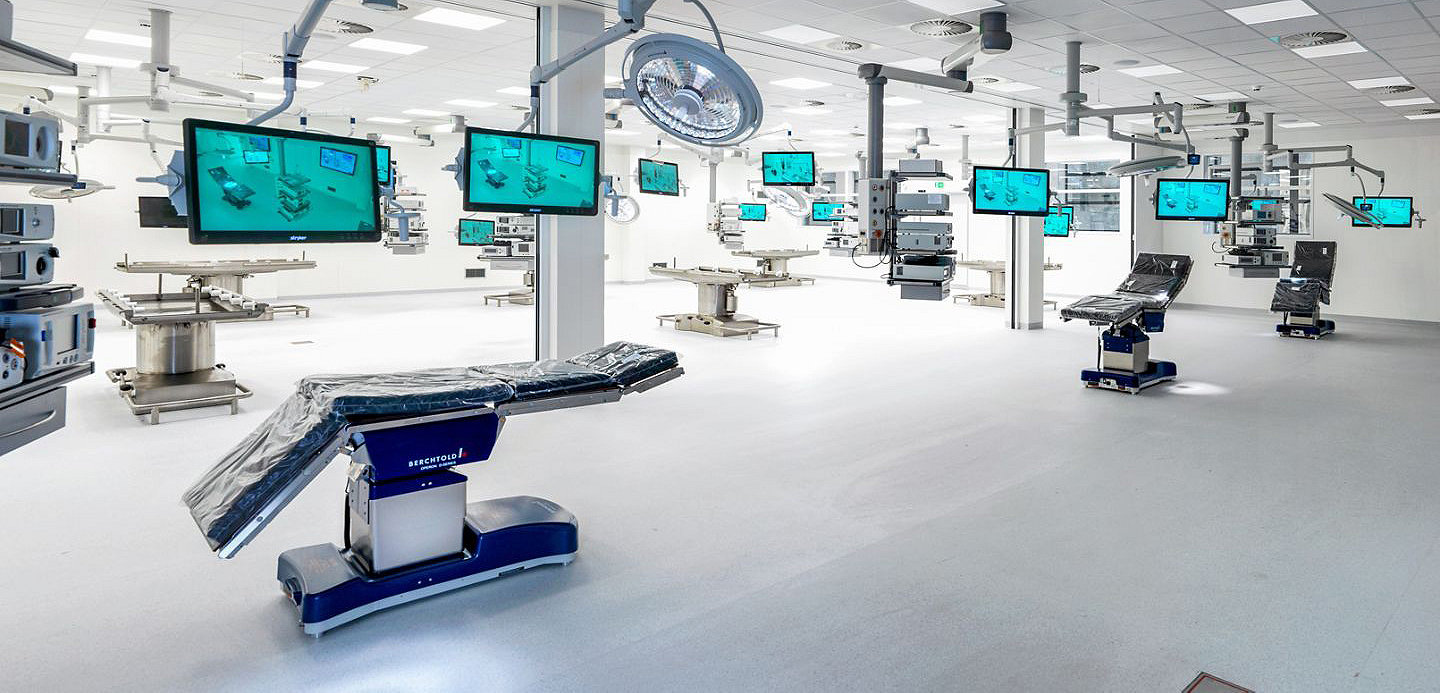
Amsterdam Skills Centre
Together with the Amsterdam University Medical Center (AMC) and Vrije University (VU), Stryker is a proud partner of the Amsterdam Skills Centre, which opened its doors on 4th February 2019.
The Amsterdam Skills Centre provides a state of the art operating theater, with 12 operating tables providing training of complex procedures on preserved human tissue. A digital learning environment is supported in the 3700m2 building, which has conference rooms for up to 200 people.
European Association of Neurosurgical Societies (EANS)
The European Association of Neurosurgical Societies is a professional community to enhance the quality of patient care through training, education and research. With 39 member societies, 3 affiliated societies and over 1800 individual members, it is the largest neurosurgical society in Europe.
As a long standing industry partner, Stryker has been supporting EANS courses on a yearly basis.
*subject to compliance approval and MedTech Europe guidelines.
2019-22548

.pdf.thumb.319.319.png)