ATLAS IDE Study Results
Treat anterior and posterior aneurysms with confidence
The ATLAS IDE studies have shown excellent results from the largest pivotal data to date with high complete occlusion and low complication rates using the Neuroform Atlas™ Stent System.
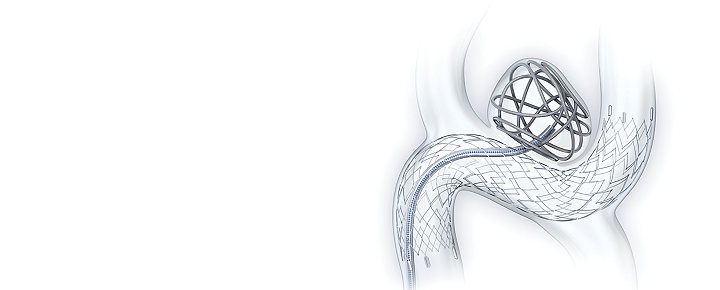
The Atlas anterior and posterior IDE studies are the largest of their kind to date, with 298 patients having diverse anatomical locations.
| Anterior n=182 |
Posterior n=116 |
|
| Primary efficacy endpoint¹ | 84.7% | 76.7% |
| Primary safety endpoint² | 4.4% | 4.3% |
| Retreatment³ | 3.8% | 7.8% |
1. Primary efficacy endpoint complete aneurysm occlusion (Raymond-Roy class 1) on 12-month angiography, in the absence of retreatment or parent artery stenosis (>50%) at the target location.
2. Primary safety endpoint of any major ipsilateral stroke or neurological death within 12 months.
3. Retreatment rate.
Atlas Posterior: Jankowitz BT, Jadhav AP, Gross B, et al, Pivotal trial of the Neuroform Atlas stent for treatment of posterior circulation aneurysms: one-year outcomes, Journal of NeuroInterventional Surgery 2022;14:143-148.
Atlas Anterior: Zaidat OO, Hanel RA, Sauvageau EA, et al. Pivotal Trial of the Neuroform Atlas Stent for Treatment of Anterior Circulation Aneurysm. Stroke. 2020; 51:2087 -2094.
"Atlas remains near and dear to my heart… It was designed to be simple to use and highly effective, with pinpoint accuracy and the lowest metal-to-artery ratio of any device on the US market."
Dr. Brian Jankowitz
This document is intended solely for the use of healthcare professionals.
A physician must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Stryker does not dispense medical advice and recommends that physicians be trained in the use of any particular product before using it in a procedure. The information presented is intended to demonstrate the breadth of Stryker product offerings. A physician must always refer to the package insert, product label and/or instructions for use before using any Stryker product. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your Stryker representative if you have questions about the availability of Stryker products in your area.
Stryker or its affiliated entities own, use, or have applied for the following trademarks or service marks: Neuroform Atlas, Stryker. All other trademarks are trademarks of their respective owners or holders.
The absence of a product, feature, or service name, or logo from this list does not constitute a waiver of Stryker’s trademark or other intellectual property rights concerning that name or logo.
AP005113 v1.0
