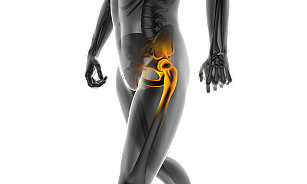
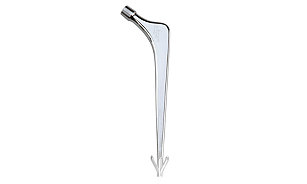
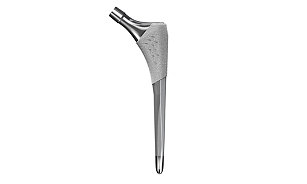
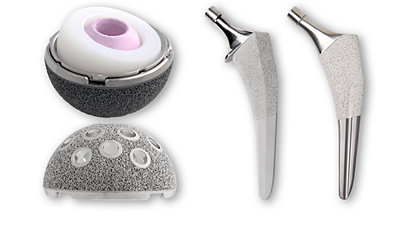
Hip
Implants
We offer market-leading hip replacement implants for total hip arthroplasty, including our primary and revision portfolios designed to offer you a wide variety of implants, instrumentation and muscle-sparing surgical approach options.
GSNPS-WC-33_27568, JR-GSNPS-PHOT-595035