Unique Device Identification (UDI)
The U.S. Food and Drug Administration (FDA) created unique device identification, often abbreviated UDI, a rule that requires medical device manufacturers to update their products with a unique device identifier that includes both device and production identifiers (such as expiration date and lot or serial number).

Download all items (Excel file of Stryker's Global Trade Item Numbers, new GTINs updated daily as new GTINs are registered with the FDA), ortho kit scan sheets (updated daily as GTINs are registered with the FDA), ortho kit list cover sheet (updated quarterly) and patient label list (updated quarterly). For questions regarding the GTIN download file, please email GTINDownloadReport@stryker.com.
The U.S. Food and Drug Administration (FDA) created unique device identification, often abbreviated UDI, a rule that requires medical device manufacturers to label their products, and in some cases, direct product markings, with both a unique device identifier and production identifiers (such as expiration date and lot or serial number). The FDA has also created a database called the Global UDI Database (GUDID) to which manufacturers upload product data and that is searchable by the public.
The FDA’s intent is to reduce medical errors and more quickly identify medical devices in the case of adverse events or recalls, in addition to providing an accessible source of definitive device identification information. Scanning barcodes containing standardized product information not only streamlines recall management, but also offers hospitals the opportunity to better manage inventory and integrate standardized product information into their electronic medical records.
The timeline for our implementation of GS1 barcodes on labels is in line with the FDA’s timeline for UDI:
- Class* III - September 24, 2014
- All other implantable, life supporting, life sustaining devices - September 24, 2015
- All other Class II devices - September 24, 2016
- All other Class I and unclassified devices - September 24, 2020
*Class is the FDA medical device classification
For more information and for the full text of the rule, visit the FDA’s web site: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/default.htm

GS1 is a data standards organization that regulates many standards across industries, such as UPCs in grocery stores or ISBNs on books. The FDA accredited GS1 as an issuing agency to assign unique device identifiers (UDIs). The standards organizations provide the UDIs and also set the rules on how the barcodes containing those UDIs appear on packaging.
Three key aspects of GS1 include:
- Global Trade Item Numbers (GTINs). GTINs are the device identifier portions of the UDIs. Each device is assigned a GTIN, just as each device is assigned a catalog number, and that GTIN will appear in a barcode on its packaging down to the unit of use (the each level).
- Global Data Synchronization Network (GDSN). GDSN is the way that suppliers and providers can synchronize their item masters. GDSN is a tool that allows providers to automatically receive product data, such as the GTIN and other relevant supply chain information.
- Global Location Numbers (GLNs). Each address has its own GLN, which provides better shipping and invoicing accuracy.
We conducted a survey of our customers globally and determined that the industry was moving toward the GS1 standard. We evaluated whether we should keep both existing HIBC codes and new GTINs on the labels, but based on input from customers and key stakeholders, we determined that it was confusing to have multiple barcodes on packaging. Through this initiative, we will transition our labels from HIBC to GS1.
For more information, visit the GS1 website: http://www.gs1us.org/healthcare


We evaluated the operational requirements to support the major process changes required to implement UDI successfully and initiated the following:
- Established a formal GS1/UDI program involving key representatives from every division at Stryker.
- Made significant investments in technology standardization between divisions and business units
- Aligned data definitions across manufacturing, quality and distribution functions to support the program requirements
- Created a barcode standard and a GTIN allocation standard
Here are some frequently asked questions about how the project may impact providers.
Q. Will you switch all products from HIBC to GS1 at one time?
No. All products manufactured after the UDI dates provided by the FDA will have GS1 barcodes; however, there will still be HIBC-labeled product in inventory.
Q. Will Stryker implement GDSN?
We are currently evaluating several GDSN data pools and plans to move forward with publishing product data to a GDSN. We will communicate the timelines for GDSN after we establish them.
Q. Can I order products using GTINs?
Some products may be ordered by GTINs. EDI orders for products that have a GTIN assigned must have both the GTIN and catalog number. We will also maintain the option for you to order by catalog number.
Q. How will the packaging labels change?
You will see our labels transition from a stacked HIBC linear barcode to a concatenated GS1-128 linear barcode.
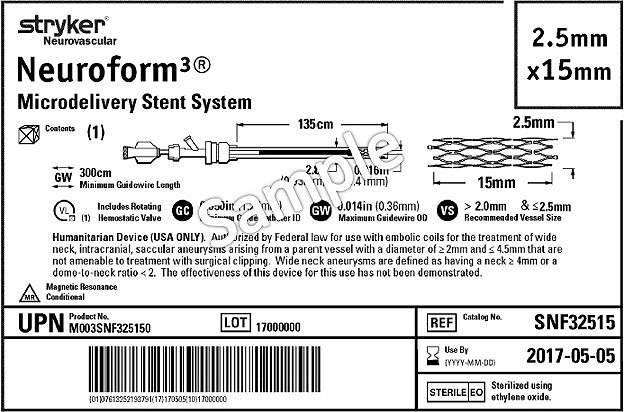
On a few packages where a concatenated GS1 barcode will not fit, you may see 2 GS1 barcodes carrying the same content.

In addition, you may also see a 2D (or data matrix) barcode.

The GS1 barcode will be at both the unit of sale and, where applicable, a single unit, or the “each” level.
Q. How will direct markings on products change?
Some products also are required to be directly marked with the UDI. In general, this applies to products that are reused and processed (sterilized, disinfected) between uses.
Q. What if my organization isn’t doing anything with GS1?
We will continue to use the catalog numbers in addition to GTINs, so you can order as you have been ordering. Nothing will change except that you will see GS1 barcodes on the packaging and your invoices and packaging slips will start to include GTINs as well as catalog numbers.
Q. My company is not in the United States. Will I see these changes?
Yes. By the end of the project, all medical devices we sell globally will have a GS1 label.
Q. Where can I find a list of Stryker's GTINs?
You can download the list on here: stryker.com/gtin.
Q. Who can I contact if I have questions about the GS1/UDI initiative?
Email questions to UDIquestions@stryker.com.
For questions regarding the GTIN download file, please email GTINDownloadReport@stryker.com.
We recognize that organizations need to improve patient safety and quality of care while lowering costs, increasing efficiencies, and adapting to new regulations. To support these initiatives, we updated our product labels to include barcodes that conform to standards created by GS1. More information can be found in the brochure at the link below.
Stryker product label transition
SYK CORP 2017-08-36
